4. (A) A medicinal chemist wished to make a series of aromatic molecules bearing a ketone and thioether group with ortho, meta, or para relationships. To achieve this, they propose using nucleophilic aromatic substitution treating the corresponding ortho, meta, and para aryl chlorides with sodium ethanethiolate (NaSEt). Predict which of these reactions will likely work and which will likely fail. Provide a mechanistic explanation why. qe NaSEt heat SEt O EtS. десете вое EtS EtS (B) Would the analogous reactions using EtMgBr instead of NaSEt be more or less likely to work? Explain why or why not. EtMgBr target molecules Et

A nucleophilic substitution reaction is a type of organic reaction where one nucleophile replaces another.
When nucleophilic substitution reaction occurs at ortho, meta, and para only ortho and para are possible not neta position.
The electron-withdrawing group's ortho or Para position to the leaving group is preferred to substitution as resonance stabilized. In contrast, meta is stabilized by the inductive, but no resonance.
The carbonyl center is the hard center and the benzene ring having Cl is the soft center and NaSEt is the soft reagent so NaSEt will attack at the Soft center. (Soft reagent will attack at the Soft center and hard reagent will attack at the Hard center)
The detailed mechanism is,
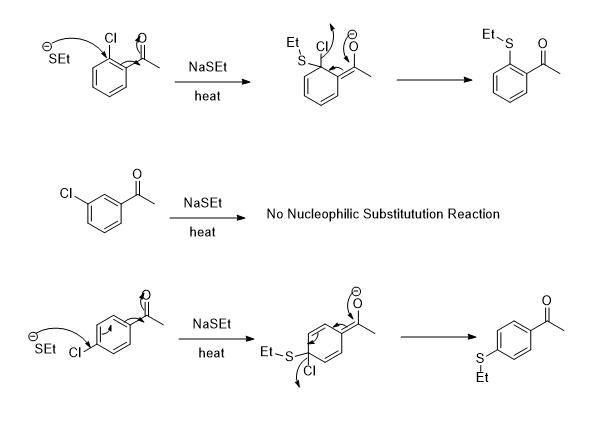
Step by step
Solved in 3 steps with 2 images









