4) The kinetic theory describes the relationship between a distribution of molecular speeds (kinetic energy) and temperature. Consider two samples of gas with the same temperature but and label the distributions of molecular one with a higher molecular mass than the other. Sketch speeds for the two samples.
4) The kinetic theory describes the relationship between a distribution of molecular speeds (kinetic energy) and temperature. Consider two samples of gas with the same temperature but and label the distributions of molecular one with a higher molecular mass than the other. Sketch speeds for the two samples.
Related questions
Question
![1 pts]
4) The kinetic theory describes the relationship between a distribution of molecular speeds
(kinetic energy) and temperature. Consider two samples of gas with the same temperature but
one with a higher molecular mass than the other. Sketch and label the distributions of molecular
speeds for the two samples.](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F6b463452-b960-4b00-bce0-3a69d9f467e2%2Fac79989b-522d-4119-90dc-7d4b8b743efa%2Fwdrst_processed.jpeg&w=3840&q=75)
Transcribed Image Text:1 pts]
4) The kinetic theory describes the relationship between a distribution of molecular speeds
(kinetic energy) and temperature. Consider two samples of gas with the same temperature but
one with a higher molecular mass than the other. Sketch and label the distributions of molecular
speeds for the two samples.
Expert Solution
Step 1
From the kinetic theory, it has been found that the molecular speeds follow the Maxwell-Boltzmann Distribution law.
According to this theory, the plot of the number of molecules with velocity is as follows:
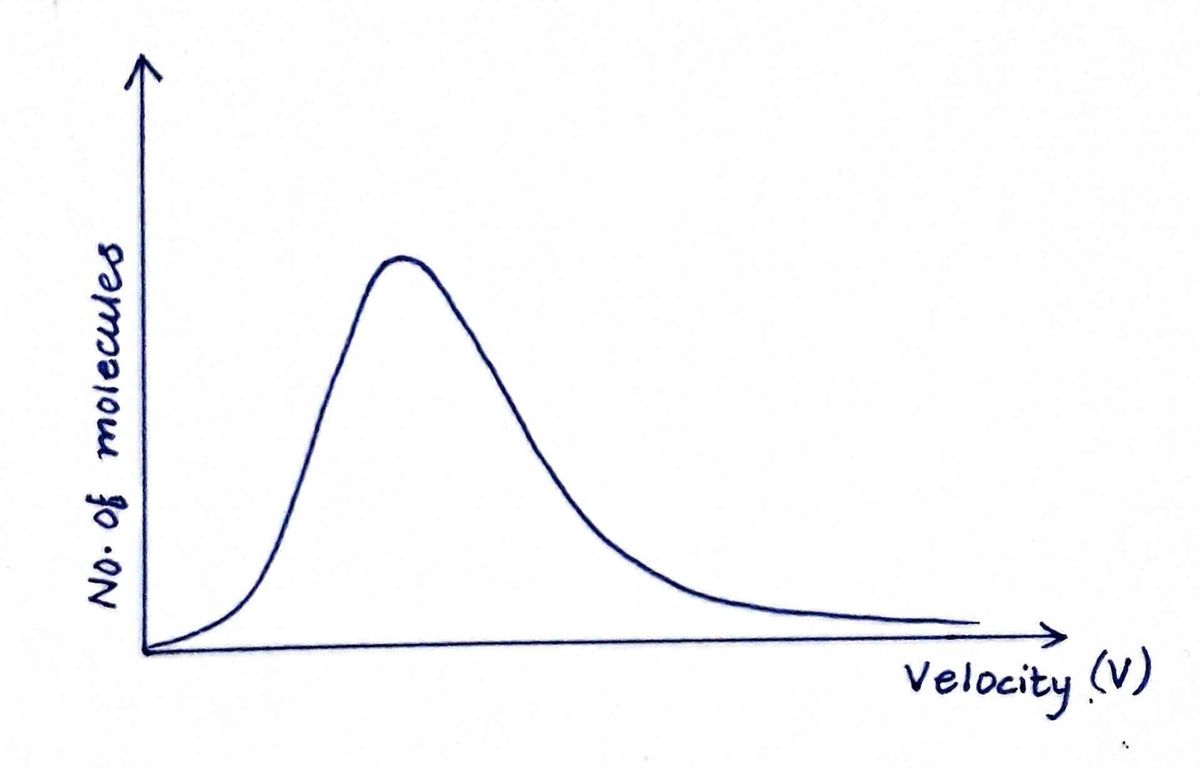
The speed distribution function f(v) {fraction of molecules having speed between v to v+dv} with velocity v, where
where M is molecular mass, v is molecular speed, R is the Universal Gas constant, and T is the temperature.
If we plot this,
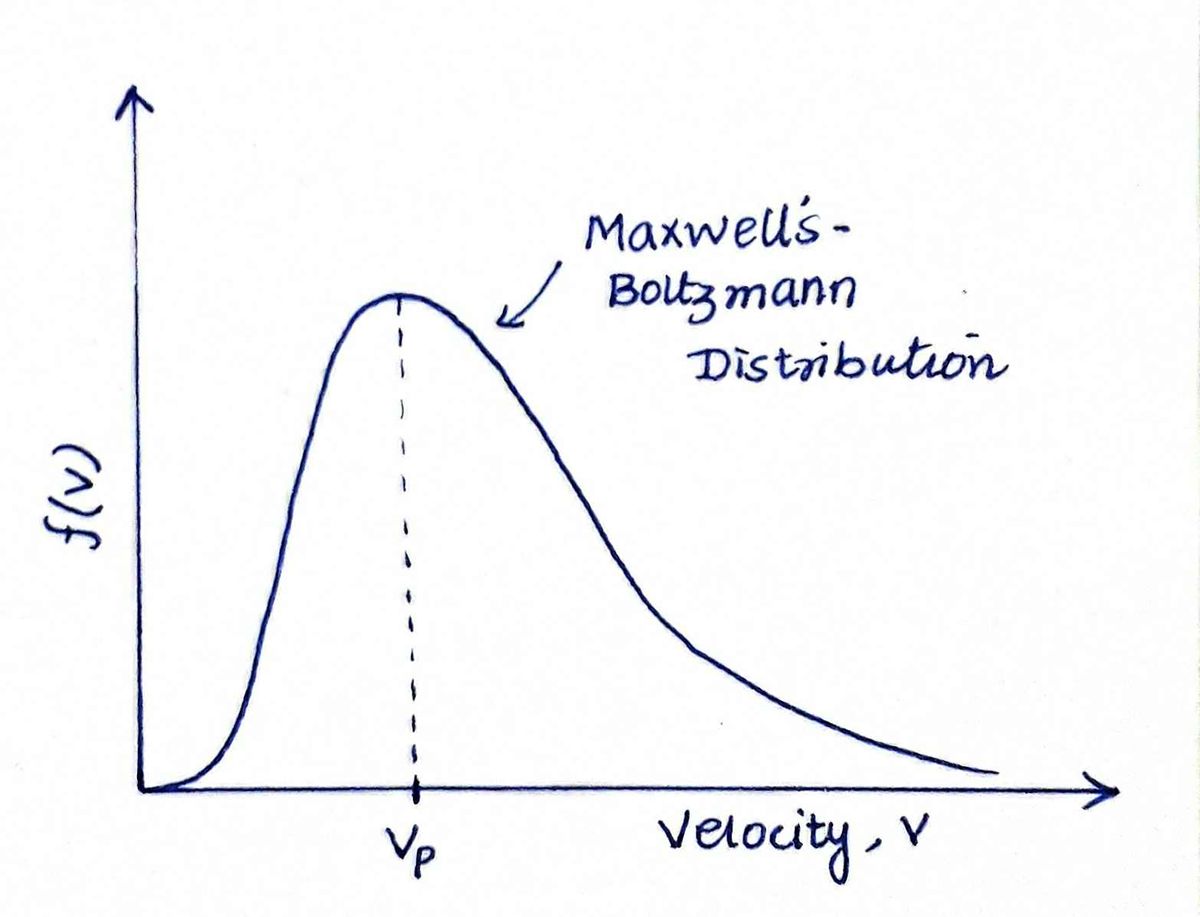
The plots look the same, the only difference is the y-axis values. In the first one, it is the number of molecules and in the second it is the fraction of molecules having speed v.
Step by step
Solved in 2 steps with 3 images
