(39 of 41) According to the reaction mechanism in Question 37, please identify all reaction intermediates. A H₂O I 1) CH3MgBr 2) H3O+ CH3 B OH X H D F OH X™ 5 CH3 E H G -H
(39 of 41) According to the reaction mechanism in Question 37, please identify all reaction intermediates. A H₂O I 1) CH3MgBr 2) H3O+ CH3 B OH X H D F OH X™ 5 CH3 E H G -H
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question

Transcribed Image Text:**Reaction Mechanism and Intermediates**
**Question Context:**
According to the reaction mechanism in Question 37, please identify all reaction intermediates.
**Reagents and Conditions:**
1) \( \text{CH}_3\text{MgBr} \)
2) \( \text{H}_3\text{O}^+ \)
**Target Product:**
The reaction leads to the formation of a tertiary alcohol (displayed as having an OH group attached to a tert-butyl group).
**Reaction Steps and Intermediates:**
1. **Intermediate A:**
- The mechanism begins with a ketone structure, where the carbonyl carbon is electrophilic.
2. **Intermediate B:**
- The \( \text{CH}_3\text{MgBr} \) performs a nucleophilic attack on the carbonyl carbon, breaking the \(C=O\) bond and forming an alkoxide ion.
3. **Intermediate C:**
- Rearrangement and stabilization of the alkoxide ion occur. The oxygen carries a negative charge represented with a lone pair, indicating its role as an anion.
4. **Intermediate D:**
- The alkoxide ion undergoes protonation facilitated by \( \text{H}_3\text{O}^+ \), leading to the formation of the alcohol intermediate.
5. **Intermediate E:**
- The carbonyl oxygen gains a proton (from \( \text{H}_3\text{O}^+ \)), resulting in an oxonium ion configuration.
6. **Intermediate F:**
- A rearrangement/transformation occurs, likely showing the pathway toward stabilization and final product formation.
7. **Intermediate G:**
- Further interaction with water (\( \text{H}_2\text{O} \)) leading to a stable hydration step that is characteristic in the final alcohol formation.
8. **Intermediate H:**
- Final deprotonation step to form the stable tertiary alcohol.
9. **Intermediate I:**
- The complete structure of the tertiary alcohol is shown. Any excess water interactions stabilize the reaction.
**Visualization:**
The diagrammatic sequence describes a multi-step reaction mechanism typical in Grignard reactions with ketones, resulting in alcohol products.
**Note:** The positions are numbered to depict electron/lone pair movements and structural changes throughout the reaction pathway.
Expert Solution
Step 1: Given reaction and mechanism
The given reaction is 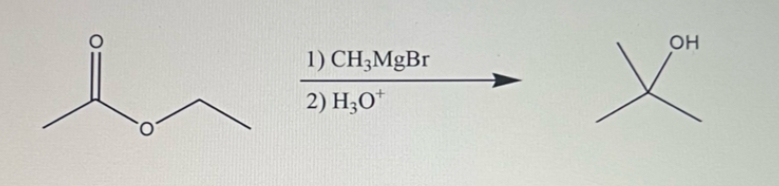
And, the given reaction mechanism also shown below
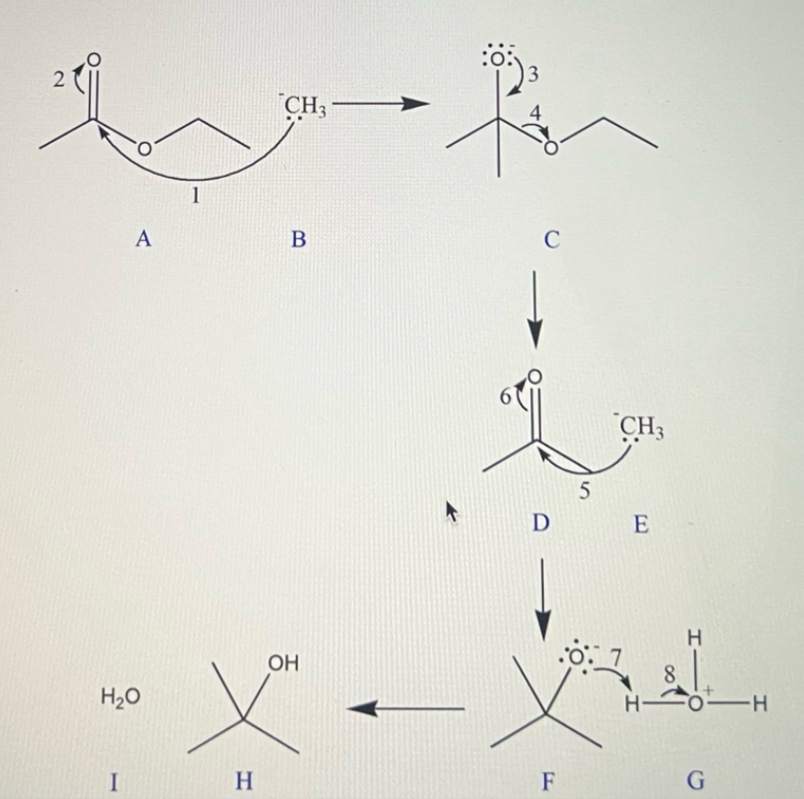
We have to determine all the reaction intermediates.
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY