3 Th To = 293 K V Room temperature T. emperature changes from Th to T during process 3. The cartoon on the right shows a piston of gas at fixed volume submerged in a ainer of room temperature water. initial state of process 3 consists of a piston containing 2 moles of a monatomic gas at Th = 373 K (boiling water temperature) and Vo consists of the gas in equilibrium
3 Th To = 293 K V Room temperature T. emperature changes from Th to T during process 3. The cartoon on the right shows a piston of gas at fixed volume submerged in a ainer of room temperature water. initial state of process 3 consists of a piston containing 2 moles of a monatomic gas at Th = 373 K (boiling water temperature) and Vo consists of the gas in equilibrium
Elements Of Electromagnetics
7th Edition
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Sadiku, Matthew N. O.
ChapterMA: Math Assessment
Section: Chapter Questions
Problem 1.1MA
Related questions
Question
Use the first image to solve two part question

Transcribed Image Text:ecreased at constanL VOlul
d
3
To
%3D
Room temperature T = 293 K
Va
V,
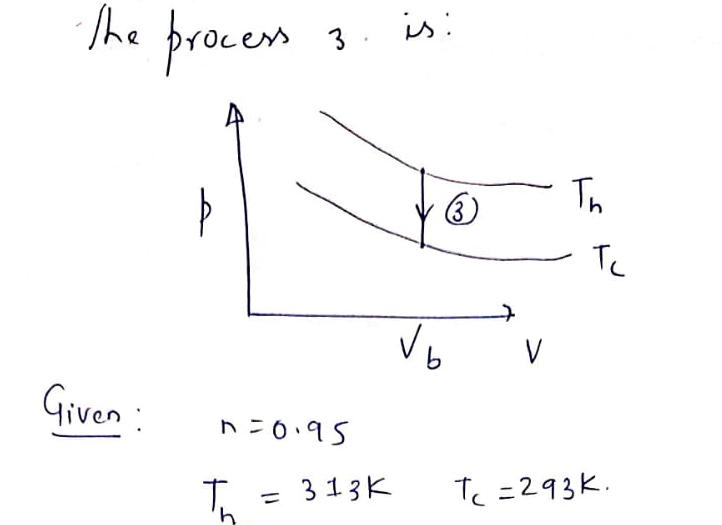
The temperature changes from Th to T, during process 3. The cartoon on the right shows a piston of gas at fixed volume submerged in a
container of room temperature water.
The initial state of process 3 consists of a piston containing 2 moles of a monatomic gas at T, = 373 K (boiling water temperature) and
he final state consists of the gas in equilibrium
3 .et placed in a reservoir of rom temperaure water cLG

Transcribed Image Text:Consider process 3 from the previous question (or in First Exam_Figures.pdf). The pressure of a gas is decreased at constant volume.
The initial state of process 3 consists of a piston containing 0.95 moles of a monatomic gas at Th= 373 K (boiling water temperature) and
%3D
volume V = 1.0 m. The piston is in contact with a bath of room temperature water at T = 293 K. The final state of the process consists of
%3D
the gas in equilibrium with the bath at 293 K.. The gas has the same volume in the initial and final states.
How much heat Q3 is transferred in process 3?
Include units in your answer.
Write your numerical answer in normal form.
Be careful to use the standard sign convention for heat transferred to the gas or to the surroundings.
QUESTION 12
Process 3 described in the previous 2 questions can be done reversibly.
O True
O False
QUESTION 13
Click Save and Submit to save and submit. Click Save All Answers to save all ansuers.
arch
Expert Solution
Step 1
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Elements Of Electromagnetics
Mechanical Engineering
ISBN:
9780190698614
Author:
Sadiku, Matthew N. O.
Publisher:
Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:
9780134319650
Author:
Russell C. Hibbeler
Publisher:
PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:
9781259822674
Author:
Yunus A. Cengel Dr., Michael A. Boles
Publisher:
McGraw-Hill Education

Elements Of Electromagnetics
Mechanical Engineering
ISBN:
9780190698614
Author:
Sadiku, Matthew N. O.
Publisher:
Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:
9780134319650
Author:
Russell C. Hibbeler
Publisher:
PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:
9781259822674
Author:
Yunus A. Cengel Dr., Michael A. Boles
Publisher:
McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:
9781118170519
Author:
Norman S. Nise
Publisher:
WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:
9781337093347
Author:
Barry J. Goodno, James M. Gere
Publisher:
Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:
9781118807330
Author:
James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:
WILEY