Ideal and Real Gases
Ideal gases obey conditions of the general gas laws under all states of pressure and temperature. Ideal gases are also named perfect gases. The attributes of ideal gases are as follows,
Gas Laws
Gas laws describe the ways in which volume, temperature, pressure, and other conditions correlate when matter is in a gaseous state. The very first observations about the physical properties of gases was made by Robert Boyle in 1662. Later discoveries were made by Charles, Gay-Lussac, Avogadro, and others. Eventually, these observations were combined to produce the ideal gas law.
Gaseous State
It is well known that matter exists in different forms in our surroundings. There are five known states of matter, such as solids, gases, liquids, plasma and Bose-Einstein condensate. The last two are known newly in the recent days. Thus, the detailed forms of matter studied are solids, gases and liquids. The best example of a substance that is present in different states is water. It is solid ice, gaseous vapor or steam and liquid water depending on the temperature and pressure conditions. This is due to the difference in the intermolecular forces and distances. The occurrence of three different phases is due to the difference in the two major forces, the force which tends to tightly hold molecules i.e., forces of attraction and the disruptive forces obtained from the thermal energy of molecules.
![79
13) A solution was made by dissolving 4.05 g of a solute in 130:9 g of acetone. The solution boiled at 56.58°
C. The boiling point of pure acetone is 55.95° C, and the Ks-1.71° C/m. What is the molecular weight
of the solute?
A) 80.3
B) 105
C) 12.7
D) 140
14) The decomposition of N₂Os(g)-NO2(g) + NO3(g) proceeds as a first order reaction with a half-life of
30.0 seconds at a certain temperature. If the initial concentration [N:Oslo 0.400 M, what is the
concentration after 150 seconds?
A) 0.400 M
B) 0.100 M
C) 0.025 M
D) 0.013 M
15) Which equation below best gives the concentration of N₂Os versus time in the previous question Q14?
A) [N₂0s] ([N2Oslo)/t12 B) [N20₁] - kt
C) [N20s]-[N2Osloe D) 1/[N20s] =1/[N20s]+ kt
T
16) The rate constant for the first order reaction A---> B+C is k-2.2 x 10 min-¹ at 57 K. What is the
half-life for this reaction at 57 K?
A) 9.1 min
D) 1200 min
B) 32 min
17) Calculate AH° for the reaction: 2C) + 2H₂O) --->
->
C(s) + H₂O
H2(g) + CO2(g)
CH4) + H₂O(g)
(1) CO(g) + H₂(g)
(2) CO(g) + H₂O(g) --->
(3) CO(g) + 3H2(g) --->
C) 64 min
CH4(g) + CO2(g)
AH-150 kJ
AH-41kJ
ΔΗ°==-206}J
A) 53 kJ
B) 116 kJ
C) -372 kJ
D)-116 kJ
18) When a strong acid is titrated with a weak base, the pH at the equivalence point is ALWAYS
A) <7
B) 7
9>7
D) <1
19) Which is the weakest intermolecular force among a group of molecules of comparable molar mass?
A) Dipole-dipole
B) London
C) Ionic bonding
D) Hydrogen bonding
20)
Given: N2(g) + 3 H₂(g) <-> 2 NH3(g)
At equilibrium at a certain temperature, the concentration of NH3(g), H₂(g) and N₂(g) are 0.490 M, 1.41 M
and 0.14 M, respectively. Calculate the value of Ke for this reaction.
A) 1.26
B) 3.02
C) 0.612
D) 1.96
21) Which one of these statements about strong base is true
A) Strong bases produce solutions with a higher pH than weak bases
B) Bases have only the OH group in their structure
C) Strong bases are very concentrated acids
D) Strong bases are 100% ionized in water
22) Consider the reaction represented by the equation: Fe³+ (aq) + SCN- (aq) → FeSCN²+ (aq)
6.00 M Fe³+ (aq) and 10.0 M SCN (aq) are mixed at a certain temperature and at equilibrium
the concentration of FeSCN2+ (aq) is 2.50 M.
A) 295x10-3
B) 303 x104
D) 95 x10³
C) 250x104
23) Consider the following reaction: 4 PCB(g)-> P4(g) + 6 Cl2(g)
If the initial concentration of PC13(g) is 3.0 M, and "x" is the equilibrium concentration of P4(g), what is the
correct equilibrium relation?
A) Kc = 6x7
B) Kc=6x7/(3.0-x)+
D) Kc=x7/(3.0-x)*
C) Kc = (x)(6x)/(3.0-4x)*
24) What is the density of NH3 at 5 atm pressure and a temperature of 35°C?
C) 0.980 g/L
B) 3.36 g/L
A) 16.6 g/L
25) What is the pH of 80.0 M aqueous solution of NH3 (ammonia). It's Kb = 1.8 x 10³.
B) 1.98
A)12.6
C) 0.778
D) 1.39 g/L
D) 0.0104
CAS](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F48079d7c-7248-4f29-b2fc-02e5764ca6aa%2Fab3cc8da-af71-4737-bb10-b5346af452b1%2Fa8t4cos_processed.jpeg&w=3840&q=75)
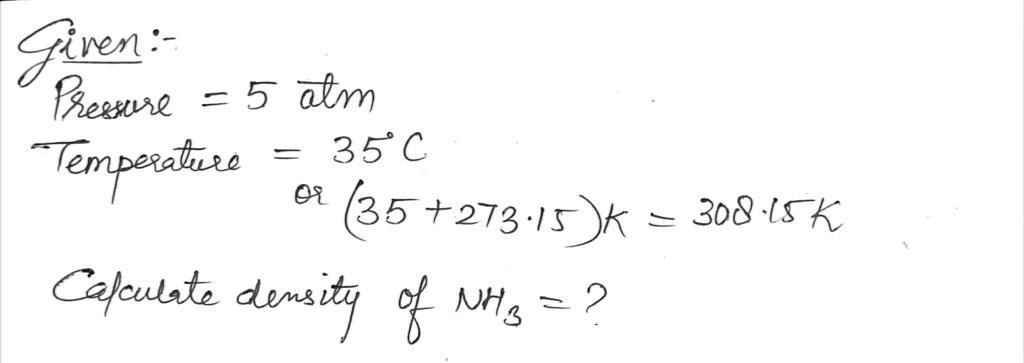
Step by step
Solved in 2 steps with 2 images









