2.4 The mercury in 0.1042 g sample was precipitated with excess of paraperiodic acid, H51O6: 5Hg²+ + 2H51O6 →→ Hg5(106)2 + 10H+, The precipitate was filtered, washed free of precipitating agent, dried and weighed and 0.5212 g was recovered. Calculate the percentage of mercury chloride in the sample.
2.4 The mercury in 0.1042 g sample was precipitated with excess of paraperiodic acid, H51O6: 5Hg²+ + 2H51O6 →→ Hg5(106)2 + 10H+, The precipitate was filtered, washed free of precipitating agent, dried and weighed and 0.5212 g was recovered. Calculate the percentage of mercury chloride in the sample.
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter17: Principles Of Chemical Reactivity: Other Aspects Of Aqueous Equilibria
Section: Chapter Questions
Problem 113SCQ: Suggest a method for separating a precipitate consisting of a mixture of solid CuS and solid...
Related questions
Question

Transcribed Image Text:2.4
The mercury in 0.1042 g sample was precipitated with excess of paraperiodic
acid, H5lO6: 5Hg²+ + 2H51O6 Hg5(106)2 + 10H+, The precipitate was filtered,
washed free of precipitating agent, dried and weighed and 0.5212 g was
recovered. Calculate the percentage of mercury chloride in the sample.
Expert Solution
Step 1
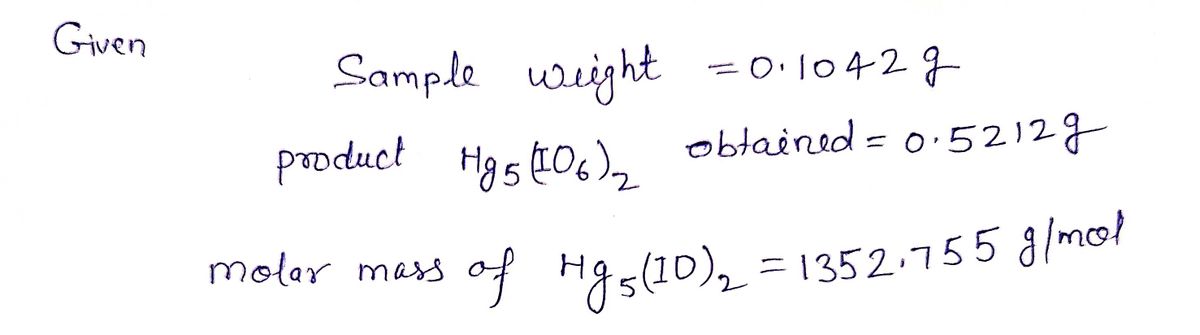
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning