2) Label the least favorable conformation and most favorable conformation of the substituted cyclohexane below. Explain your choices in 1-2 sentences or less. H₂C H3C- H3C F OH CH3 H3 CH3 XCH₂ -- F H3C CH3 CH3 OH H3C CH3 F CH₂ CH3 -OH

A-First A Chair conformation is most stable because tert-butyl group is present at Equatorial Position means no steric hinderance or no butane gauch interaction with adjacent carbon.
C- C Chair conformation is more stable than B and less stable than A because tert-butyl group is present at axial Position means steric hinderance or butane gauch interaction with adjacent carbon and tert-butyl group.
B-We Know Boat conformation is less stable than chair conformation and conformation B is least stable because tert-butyl group is present at axial Position means lot of steric hinderance or butane gauch interaction with adjacent carbon and tert-butyl group with boat conformation also.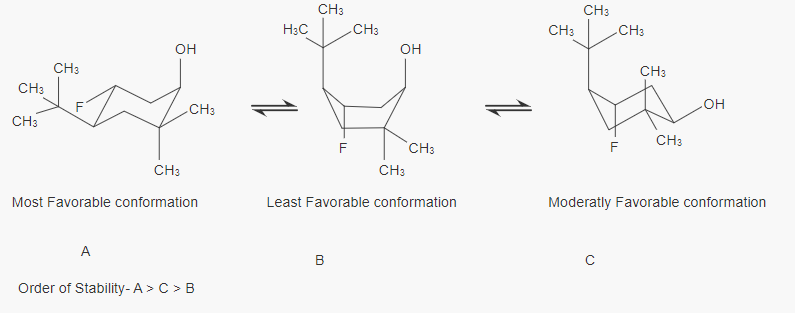
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images









