Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
1e.

Transcribed Image Text:**Subject: Organic Chemistry - Nucleophilic Substitution Reactions**
**Topic: Ranking Molecules by SN2 Reaction Rate**
**Objective:** For each of the following sets of molecules, rank them in order of increasing rate of SN2 reaction. (1 = slowest; 3 = fastest)
---
**a. Molecule Set:**
1. **Structure 1:** A benzene ring with a negative charge on the nitrogen (an aniline derivative).
2. **Structure 2:** A simple aliphatic chain with a negatively charged nitrogen (primary amine).
3. **Structure 3:** An amide with a methyl group.
**b. Molecule Set:**
1. **Structure 1:** A sulfur atom doubly bonded to an adjacent alpha-carbon, with three methyl groups (sodium salt of a thiolate).
2. **Structure 2:** A straight-chain alkane with an oxygen carrying a negative charge (sodium alkoxide).
3. **Structure 3:** A tertiary carbon center with a lithium counterion.
**c. Molecule Set:**
1. **Structure 1:** Chloromethyl benzene (benzyl chloride).
2. **Structure 2:** Propan-2-yl chloride with two methyl groups.
3. **Structure 3:** A cyclopentene with a chloride substituent.
**d. Molecule Set:**
1. **Structure 1:** A simple alkenyl chloride.
2. **Structure 2:** A similar alkene structure with a bromine substituent instead of chlorine.
3. **Structure 3:** An iodine substituent on a tertiary carbon center with additional methyl groups.
**e. Molecule Set:**
1. **Structure 1:** A trifluoromethylsulfonate ester on a cyclopentane ring.
2. **Structure 2:** A cyclopentane ring with a bromine substituent.
3. **Structure 3:** A cyclopentylamine.
---
**Explanation:**
The SN2 reaction is a bimolecular nucleophilic substitution that is influenced by several factors including sterics, leaving group ability, and nucleophile strength. Generally, primary substrates react faster than secondary, which are faster than tertiary, due to less steric hindrance. Better leaving groups (such as iodide and bromide) also lead to faster reactions. Additionally, stronger nucleophiles (such as alkoxides and
Expert Solution
Step 1
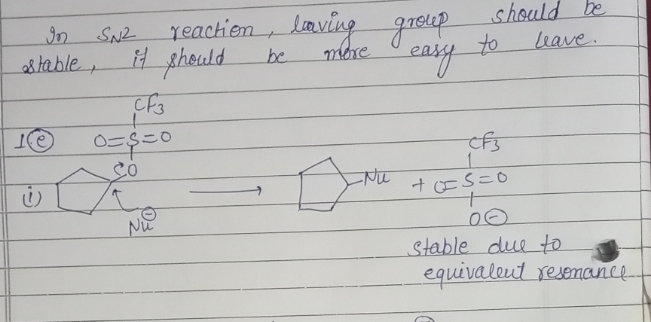
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY