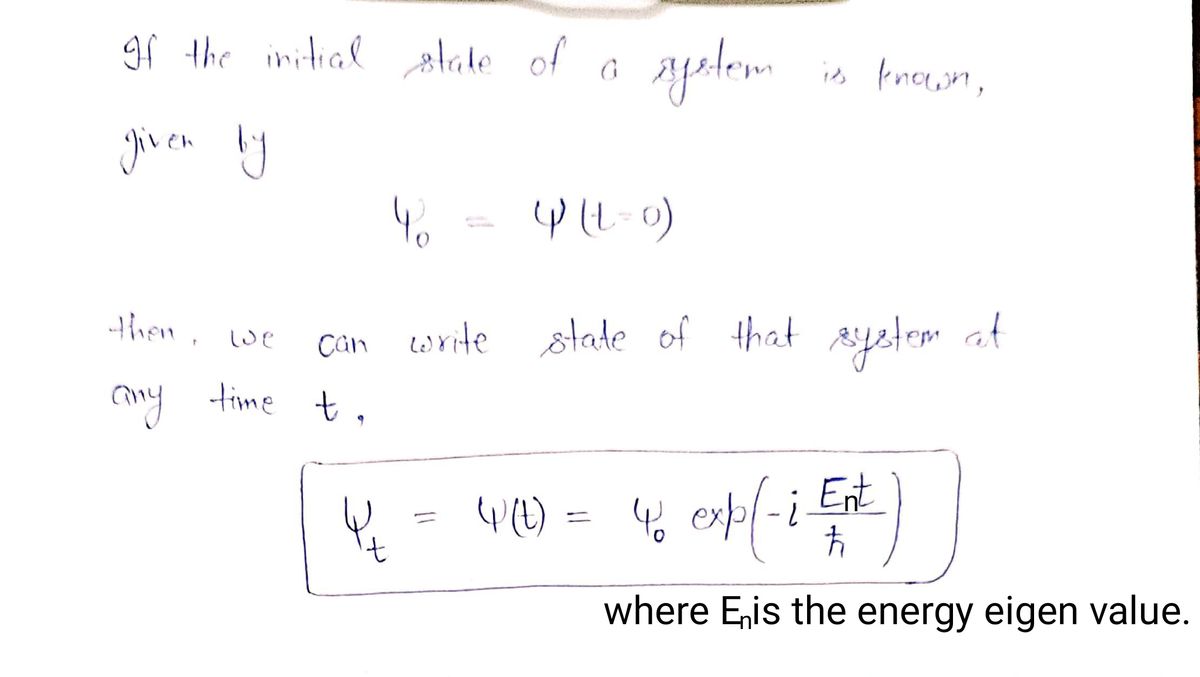
18. If the initial state of a system is known, given by y(t = 0) y(t) = where Wn= and Cn= Hon=
18. If the initial state of a system is known, given by y(t = 0) y(t) = where Wn= and Cn= Hon=
Related questions
Question

Transcribed Image Text:18. If the initial state of a system is known, given by Y(t = 0)
Y(t)=
where
and
Wn =
Cn=
HPn=
=
Expert Solution
Step 1
Step by step
Solved in 2 steps with 2 images
