Last month, Jim purchased $10,000 of US Treasury Bonds (their face value was $10,000). These bonds have a 30-year maturity period, and they pay 1.5% interest every three months (i.e. the APR is 6% and Jim receives a check for $150 every three months). But interest rates for similar securities have since risen to a 7% APR because of interest rate increases by the Federal Reserve Board. In view of the interest rate increases to 7%, what is the current value of Jim's bonds? 7% = 1.75 or 0.00175% Pmt 1 4(quarter) -> Present Value of Annuity → PV 50 (1 (1 - (1 +/- 1)) r 150 1- PV = 1 (1+0.0175) 0.00175 = 146.4893 150 1 (1 - PV₂ = = 296.793 PV3 = (1+0.0175)² 0.0175 150 (1- (1+0.0175)³) 0.0175 = 429.1239 TPV = PV₁ + PV2 + PV3 = $872.41
Last month, Jim purchased $10,000 of US Treasury Bonds (their face value was $10,000). These bonds have a 30-year maturity period, and they pay 1.5% interest every three months (i.e. the APR is 6% and Jim receives a check for $150 every three months). But interest rates for similar securities have since risen to a 7% APR because of interest rate increases by the Federal Reserve Board. In view of the interest rate increases to 7%, what is the current value of Jim's bonds? 7% = 1.75 or 0.00175% Pmt 1 4(quarter) -> Present Value of Annuity → PV 50 (1 (1 - (1 +/- 1)) r 150 1- PV = 1 (1+0.0175) 0.00175 = 146.4893 150 1 (1 - PV₂ = = 296.793 PV3 = (1+0.0175)² 0.0175 150 (1- (1+0.0175)³) 0.0175 = 429.1239 TPV = PV₁ + PV2 + PV3 = $872.41
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question
is this right?

Transcribed Image Text:Last month, Jim purchased $10,000 of US Treasury Bonds (their face value was $10,000). These bonds
have a 30-year maturity period, and they pay 1.5% interest every three months (i.e. the APR is 6% and
Jim receives a check for $150 every three months). But interest rates for similar securities have since
risen to a 7% APR because of interest rate increases by the Federal Reserve Board. In view of the
interest rate increases to 7%, what is the current value of Jim's bonds?
7%
4(quarter)
= 1.75 or 0.00175%
1
Pmt (1
(1+r)",
r
Present Value of Annuity → PV
PV =
PV2
=
PV3
=
1
150 (1 − (1 + 0.0175).
0.00175
1
(150 (1 − (1 + 0.0175)²
-
0.0175
1
150 (1 − (1 + 0.0175)³
0.0175
= 146.4893
=
296.793
= 429.1239
TPV = PV₁ + PV2 + PV3 = $872.41
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 18 images

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
i am having a hard time coming up with the same calculations. can u plz expand
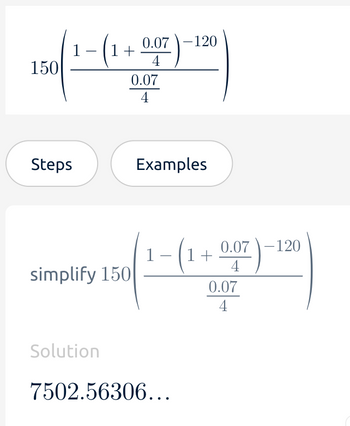
Transcribed Image Text:150
1
-
(1+0.07)-
4
0.07
4
-120
Steps
Examples
0.07-120
1+
simplify 150
4
0.07
4
Solution
7502.56306...
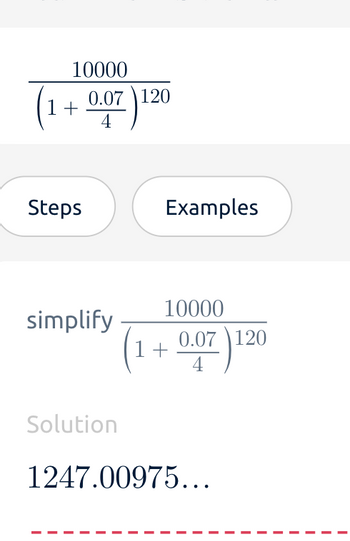
Transcribed Image Text:10000
(1 + 0.07) 120
Steps
Examples
simplify
Solution
10000
(1 + 0.07) 120
1247.00975...
Solution
Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The