14. How molecules must be oriented to each other in order for a reaction to occur is part of the a. reaction rate b. activation energy c. reaction mechanism d. determination if a reaction is spontaneous
14. How molecules must be oriented to each other in order for a reaction to occur is part of the
According to the collision theory, molecules must collide with each other with sufficient kinetic energy (greater than the activation energy) to form the product and molecules also need to collide with the right orientation so that bonds can break and re-form in the necessary manner.
How often the collision will occur or how effective the collision will be (in terms of energy) depends on the reaction rate or activation energy. For the correct orientation, knowledge of reaction mechanism is necessary to determine whether the reaction will take place or not.
For example- In the reaction of ethene with HCl,
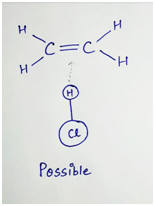
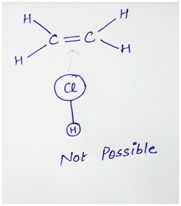
Collision second is unsuccessful (or not possible). This is because, the double bond has high concentration of negative charge around it. The approaching Cl is also negative charge due to dipole created by the electronegativity difference between H and Cl. Thus there is repulsion between the ethene molecule and Cl atom which causes the molecule to bounce off. Thus for the proper orientation of the molecule in a reaction, reaction mechanism must be known.
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images









