1:39 ll LTE Question 15 of 20 Submit nsider the balanced chemical reaction below. ow many grams of carbon monoxide are required to react with 6.4 g of iron(III) oxide? Fe2O3(s) + 3 CO(g) → 2 Fe(s) + 3 CO2(g) 1 2 Based on the set up your table from the previous step, determine the grams of carbon monoxide required. massco = 5 RESET 6.4 3.2 19 0.56 1.1 1.7 2.2 3.4 7.6
1:39 ll LTE Question 15 of 20 Submit nsider the balanced chemical reaction below. ow many grams of carbon monoxide are required to react with 6.4 g of iron(III) oxide? Fe2O3(s) + 3 CO(g) → 2 Fe(s) + 3 CO2(g) 1 2 Based on the set up your table from the previous step, determine the grams of carbon monoxide required. massco = 5 RESET 6.4 3.2 19 0.56 1.1 1.7 2.2 3.4 7.6
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
How to solve
![**Stoichiometry Problem: Iron(III) Oxide and Carbon Monoxide Reaction**
**Question 15 of 20**
**Problem Statement:**
Consider the balanced chemical reaction below. How many grams of carbon monoxide are required to react with 6.4 g of iron(III) oxide?
\[ \text{Fe}_2\text{O}_3(s) + 3 \text{CO}(g) \rightarrow 2 \text{Fe}(s) + 3 \text{CO}_2(g) \]
**Task:**
Based on your knowledge of stoichiometry, set up the table below to determine the minimum number of moles of CO required to react with the Fe₂O₃ and then determine the amounts after the reaction goes to completion.
**Stoichiometry Table:**
| | Fe₂O₃(s) | 3 CO(g) | 2 Fe(s) | 3 CO₂(g) |
|---------------|------------------|----------------|----------------|---------------|
| Before (mol) | | 0.12 | 0 | 0 |
| Change (mol) | | -0.080 | 0.080 | 0.12 |
| After (mol) | | 0.040 | 0.080 | 0.12 |
**Additional Calculations:**
- (Boxes highlighted with numerical options, likely for input of calculated values)
**Buttons:**
- [RESET]
**Instructions:**
Tap here or pull up for additional resources.
**Note:**
The problem requires calculating the stoichiometric quantities of carbon monoxide needed to react with a given mass of iron(III) oxide using the provided balanced equation. The table assists in visualizing the mole changes during the reaction.](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F1e4f7fd3-dbae-49c8-b999-a719c9747eb1%2Fd1b9a831-aa38-4594-bacd-66a6293564f7%2F733z8p_processed.png&w=3840&q=75)
Transcribed Image Text:**Stoichiometry Problem: Iron(III) Oxide and Carbon Monoxide Reaction**
**Question 15 of 20**
**Problem Statement:**
Consider the balanced chemical reaction below. How many grams of carbon monoxide are required to react with 6.4 g of iron(III) oxide?
\[ \text{Fe}_2\text{O}_3(s) + 3 \text{CO}(g) \rightarrow 2 \text{Fe}(s) + 3 \text{CO}_2(g) \]
**Task:**
Based on your knowledge of stoichiometry, set up the table below to determine the minimum number of moles of CO required to react with the Fe₂O₃ and then determine the amounts after the reaction goes to completion.
**Stoichiometry Table:**
| | Fe₂O₃(s) | 3 CO(g) | 2 Fe(s) | 3 CO₂(g) |
|---------------|------------------|----------------|----------------|---------------|
| Before (mol) | | 0.12 | 0 | 0 |
| Change (mol) | | -0.080 | 0.080 | 0.12 |
| After (mol) | | 0.040 | 0.080 | 0.12 |
**Additional Calculations:**
- (Boxes highlighted with numerical options, likely for input of calculated values)
**Buttons:**
- [RESET]
**Instructions:**
Tap here or pull up for additional resources.
**Note:**
The problem requires calculating the stoichiometric quantities of carbon monoxide needed to react with a given mass of iron(III) oxide using the provided balanced equation. The table assists in visualizing the mole changes during the reaction.
![**Question 15 of 20**
**Consider the balanced chemical reaction below. How many grams of carbon monoxide are required to react with 6.4 g of iron(III) oxide?**
\[ \text{Fe}_2\text{O}_3(\text{s}) + 3 \text{CO}(\text{g}) \rightarrow 2 \text{Fe}(\text{s}) + 3 \text{CO}_2(\text{g}) \]
**Based on the setup of your table from the previous step, determine the grams of carbon monoxide required.**
\[ \text{mass}_{\text{CO}} = \underline{\hspace{2cm}} \text{g} \]
**Options Available**:
0, 6.4, 3.2, 19
0.56, 1.1, 1.7, 2.2
3.4, 7.6
**Navigation and Interaction Details:**
- A progress bar is displayed with question number 15 highlighted.
- A reset button is provided for resetting the question.
- Instruction available to tap or pull up for additional resources.
**Explanation of Interactive Elements:**
- This question involves calculating the mass of carbon monoxide needed for a chemical reaction involving iron(III) oxide.
- Users are prompted to use a table setup from a previous step and select the correct answer from multiple-choice options.](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F1e4f7fd3-dbae-49c8-b999-a719c9747eb1%2Fd1b9a831-aa38-4594-bacd-66a6293564f7%2Flalt15o_processed.png&w=3840&q=75)
Transcribed Image Text:**Question 15 of 20**
**Consider the balanced chemical reaction below. How many grams of carbon monoxide are required to react with 6.4 g of iron(III) oxide?**
\[ \text{Fe}_2\text{O}_3(\text{s}) + 3 \text{CO}(\text{g}) \rightarrow 2 \text{Fe}(\text{s}) + 3 \text{CO}_2(\text{g}) \]
**Based on the setup of your table from the previous step, determine the grams of carbon monoxide required.**
\[ \text{mass}_{\text{CO}} = \underline{\hspace{2cm}} \text{g} \]
**Options Available**:
0, 6.4, 3.2, 19
0.56, 1.1, 1.7, 2.2
3.4, 7.6
**Navigation and Interaction Details:**
- A progress bar is displayed with question number 15 highlighted.
- A reset button is provided for resetting the question.
- Instruction available to tap or pull up for additional resources.
**Explanation of Interactive Elements:**
- This question involves calculating the mass of carbon monoxide needed for a chemical reaction involving iron(III) oxide.
- Users are prompted to use a table setup from a previous step and select the correct answer from multiple-choice options.
Expert Solution
Step 1
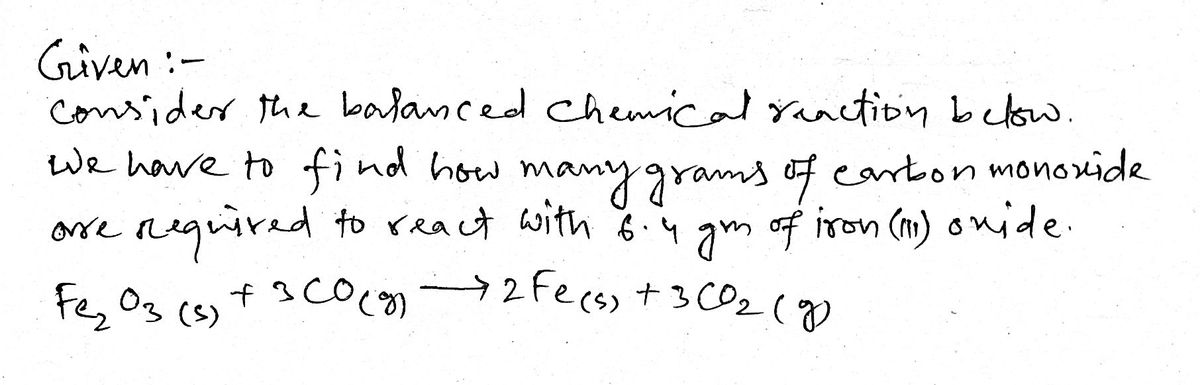
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY