13) A solution was made by dissolving 4.05 g of a solute in 1369 g of acetone. The solution boiled at 56.58 79 C. The boiling point of pure acetone is 55.95° C, and the Ks-1.71° C/m. What is the molecular weight of the solute? A) 80.3 B) 105 C) 12.7 D) 140 14) The decomposition of N₂Os(g)-NO₂(g) + NO(g) proceeds as a first order reaction with a half-life of 30.0 seconds at a certain temperature. If the initial concentration [N:Oslo 0.400 M, what is the concentration after 150 seconds? A) 0.400 M B) 0.100 M C) 0.025 M D) 0.013 M 15) Which equation below best gives the concentration of N:Os versus time in the previous question Q14? A) [N₂Os] =([N2Oslo)/t12 B) [N20s] - kt C) [N₂0s]-[N2Osloe D) 1/[N20s]=1/[N₂0s]+kt 16) The rate constant for the first order reaction A--> B+C is k-2.2 x 10³ min¹ at 57 K. What is the half-life for this reaction at 57 K? A) 9.1 min B) 32 min D) 1200 min 17) Calculate AH° for the reaction: 2C+2H₂O ---> (1) CO +Hz -> (2) CO +HzOng mà (3) CO(g) + 3H2(g) →→> C) + H₂O H₂(g) + CO2(g) CH4g) + H₂O(g) C) 64 min CH«p+COzp AH--150 kJ AH° -41kJ AH-206kJ A) 53 kJ B) 116 kJ C) -372 kJ D)-116 kJ 18) When a strong acid is titrated with a weak base, the pH at the equivalence point is ALWAYS A) <7 B) 7 97 D) <1 19) Which is the weakest intermolecular force among a group of molecules of comparable molar mass? A) Dipole-dipole B) London C) Ionic bonding D) Hydrogen bonding 20) Given: N₂(g) + 3 H₂(g) <->2 NH3(g) At equilibrium at a certain temperature, the concentration of NH3(g), H₂(g) and N₂(g) are 0.490 M, 1.41 M and 0.14 M, respectively. Calculate the value of Ke for this reaction. A) 1.26 B) 3.02 C) 0.612 D) 1.96 21) Which one of these statements about strong base is true A) Strong bases produce solutions with a higher pH than weak bases B) Bases have only the OH group in their structure C) Strong bases are very concentrated acids D) Strong bases are 100% ionized in water 22) Consider the reaction represented by the equation: Fe³+ (aq) + SCN (aq) → FeSCN²+ (aq) 6.00 M Fe³+ (aq) and 10.0 M SCN (aq) are mixed at a certain temperature and at equilibrium the concentration of FeSCN2+ (aq) is 2.50 M. A) 295x10-3 B) 303 x104 D) 95 x10-³ C) 250 x10+ 23) Consider the following reaction: 4 PCB(g)-> P4(g) + 6 Cl₂(g) If the initial concentration of PC13(g) is 3.0 M, and "x" is the equilibrium concentration of P4(g), what is the correct equilibrium relation? A) Kc=6x7 B) Kc=6x7/(3.0-x)* D) Kc=x7/(3.0-x)* C) Kc =(x)(6x)/(3.0-4x)* 24) What is the density of NH3 at 5 atm pressure and a temperature of 35°C? C) 0.980 g/L B) 3.36 g/L A) 16.6 g/L 25) What is the pH of 80.0 M aqueous solution of NH3 (ammonia). It's Kb = 1.8 x 10³. B) 1.98 A)12.6 C) 0.778 D) 1.39 g/L D) 0.0104
13) A solution was made by dissolving 4.05 g of a solute in 1369 g of acetone. The solution boiled at 56.58 79 C. The boiling point of pure acetone is 55.95° C, and the Ks-1.71° C/m. What is the molecular weight of the solute? A) 80.3 B) 105 C) 12.7 D) 140 14) The decomposition of N₂Os(g)-NO₂(g) + NO(g) proceeds as a first order reaction with a half-life of 30.0 seconds at a certain temperature. If the initial concentration [N:Oslo 0.400 M, what is the concentration after 150 seconds? A) 0.400 M B) 0.100 M C) 0.025 M D) 0.013 M 15) Which equation below best gives the concentration of N:Os versus time in the previous question Q14? A) [N₂Os] =([N2Oslo)/t12 B) [N20s] - kt C) [N₂0s]-[N2Osloe D) 1/[N20s]=1/[N₂0s]+kt 16) The rate constant for the first order reaction A--> B+C is k-2.2 x 10³ min¹ at 57 K. What is the half-life for this reaction at 57 K? A) 9.1 min B) 32 min D) 1200 min 17) Calculate AH° for the reaction: 2C+2H₂O ---> (1) CO +Hz -> (2) CO +HzOng mà (3) CO(g) + 3H2(g) →→> C) + H₂O H₂(g) + CO2(g) CH4g) + H₂O(g) C) 64 min CH«p+COzp AH--150 kJ AH° -41kJ AH-206kJ A) 53 kJ B) 116 kJ C) -372 kJ D)-116 kJ 18) When a strong acid is titrated with a weak base, the pH at the equivalence point is ALWAYS A) <7 B) 7 97 D) <1 19) Which is the weakest intermolecular force among a group of molecules of comparable molar mass? A) Dipole-dipole B) London C) Ionic bonding D) Hydrogen bonding 20) Given: N₂(g) + 3 H₂(g) <->2 NH3(g) At equilibrium at a certain temperature, the concentration of NH3(g), H₂(g) and N₂(g) are 0.490 M, 1.41 M and 0.14 M, respectively. Calculate the value of Ke for this reaction. A) 1.26 B) 3.02 C) 0.612 D) 1.96 21) Which one of these statements about strong base is true A) Strong bases produce solutions with a higher pH than weak bases B) Bases have only the OH group in their structure C) Strong bases are very concentrated acids D) Strong bases are 100% ionized in water 22) Consider the reaction represented by the equation: Fe³+ (aq) + SCN (aq) → FeSCN²+ (aq) 6.00 M Fe³+ (aq) and 10.0 M SCN (aq) are mixed at a certain temperature and at equilibrium the concentration of FeSCN2+ (aq) is 2.50 M. A) 295x10-3 B) 303 x104 D) 95 x10-³ C) 250 x10+ 23) Consider the following reaction: 4 PCB(g)-> P4(g) + 6 Cl₂(g) If the initial concentration of PC13(g) is 3.0 M, and "x" is the equilibrium concentration of P4(g), what is the correct equilibrium relation? A) Kc=6x7 B) Kc=6x7/(3.0-x)* D) Kc=x7/(3.0-x)* C) Kc =(x)(6x)/(3.0-4x)* 24) What is the density of NH3 at 5 atm pressure and a temperature of 35°C? C) 0.980 g/L B) 3.36 g/L A) 16.6 g/L 25) What is the pH of 80.0 M aqueous solution of NH3 (ammonia). It's Kb = 1.8 x 10³. B) 1.98 A)12.6 C) 0.778 D) 1.39 g/L D) 0.0104
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
Q 15 please
![79
13) A solution was made by dissolving 4.05 g of a solute in 130:9 g of acetone. The solution boiled at 56.58°
C. The boiling point of pure acetone is 55.95° C, and the Ks-1.71° C/m. What is the molecular weight
of the solute?
A) 80.3
B) 105
C) 12.7
D) 140
14) The decomposition of N₂Os(g)-NO2(g) + NO3(g) proceeds as a first order reaction with a half-life of
30.0 seconds at a certain temperature. If the initial concentration [N:Oslo 0.400 M, what is the
concentration after 150 seconds?
A) 0.400 M
B) 0.100 M
C) 0.025 M
D) 0.013 M
15) Which equation below best gives the concentration of N₂Os versus time in the previous question Q14?
A) [N₂0s] ([N2Oslo)/t12 B) [N20₁] - kt
C) [N20s]-[N2Osloe D) 1/[N20s] =1/[N20s]+ kt
T
16) The rate constant for the first order reaction A---> B+C is k-2.2 x 10 min-¹ at 57 K. What is the
half-life for this reaction at 57 K?
A) 9.1 min
D) 1200 min
B) 32 min
17) Calculate AH° for the reaction: 2C) + 2H₂O) --->
->
C(s) + H₂O
H2(g) + CO2(g)
CH4) + H₂O(g)
(1) CO(g) + H₂(g)
(2) CO(g) + H₂O(g) --->
(3) CO(g) + 3H2(g) --->
C) 64 min
CH4(g) + CO2(g)
AH-150 kJ
AH-41kJ
ΔΗ°==-206}J
A) 53 kJ
B) 116 kJ
C) -372 kJ
D)-116 kJ
18) When a strong acid is titrated with a weak base, the pH at the equivalence point is ALWAYS
A) <7
B) 7
9>7
D) <1
19) Which is the weakest intermolecular force among a group of molecules of comparable molar mass?
A) Dipole-dipole
B) London
C) Ionic bonding
D) Hydrogen bonding
20)
Given: N2(g) + 3 H₂(g) <-> 2 NH3(g)
At equilibrium at a certain temperature, the concentration of NH3(g), H₂(g) and N₂(g) are 0.490 M, 1.41 M
and 0.14 M, respectively. Calculate the value of Ke for this reaction.
A) 1.26
B) 3.02
C) 0.612
D) 1.96
21) Which one of these statements about strong base is true
A) Strong bases produce solutions with a higher pH than weak bases
B) Bases have only the OH group in their structure
C) Strong bases are very concentrated acids
D) Strong bases are 100% ionized in water
22) Consider the reaction represented by the equation: Fe³+ (aq) + SCN- (aq) → FeSCN²+ (aq)
6.00 M Fe³+ (aq) and 10.0 M SCN (aq) are mixed at a certain temperature and at equilibrium
the concentration of FeSCN2+ (aq) is 2.50 M.
A) 295x10-3
B) 303 x104
D) 95 x10³
C) 250x104
23) Consider the following reaction: 4 PCB(g)-> P4(g) + 6 Cl2(g)
If the initial concentration of PC13(g) is 3.0 M, and "x" is the equilibrium concentration of P4(g), what is the
correct equilibrium relation?
A) Kc = 6x7
B) Kc=6x7/(3.0-x)+
D) Kc=x7/(3.0-x)*
C) Kc = (x)(6x)/(3.0-4x)*
24) What is the density of NH3 at 5 atm pressure and a temperature of 35°C?
C) 0.980 g/L
B) 3.36 g/L
A) 16.6 g/L
25) What is the pH of 80.0 M aqueous solution of NH3 (ammonia). It's Kb = 1.8 x 10³.
B) 1.98
A)12.6
C) 0.778
D) 1.39 g/L
D) 0.0104
CAS](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F48079d7c-7248-4f29-b2fc-02e5764ca6aa%2F12031053-8d4c-4175-b032-010d2ecd8ea3%2Fs9v0xo9_processed.jpeg&w=3840&q=75)
Transcribed Image Text:79
13) A solution was made by dissolving 4.05 g of a solute in 130:9 g of acetone. The solution boiled at 56.58°
C. The boiling point of pure acetone is 55.95° C, and the Ks-1.71° C/m. What is the molecular weight
of the solute?
A) 80.3
B) 105
C) 12.7
D) 140
14) The decomposition of N₂Os(g)-NO2(g) + NO3(g) proceeds as a first order reaction with a half-life of
30.0 seconds at a certain temperature. If the initial concentration [N:Oslo 0.400 M, what is the
concentration after 150 seconds?
A) 0.400 M
B) 0.100 M
C) 0.025 M
D) 0.013 M
15) Which equation below best gives the concentration of N₂Os versus time in the previous question Q14?
A) [N₂0s] ([N2Oslo)/t12 B) [N20₁] - kt
C) [N20s]-[N2Osloe D) 1/[N20s] =1/[N20s]+ kt
T
16) The rate constant for the first order reaction A---> B+C is k-2.2 x 10 min-¹ at 57 K. What is the
half-life for this reaction at 57 K?
A) 9.1 min
D) 1200 min
B) 32 min
17) Calculate AH° for the reaction: 2C) + 2H₂O) --->
->
C(s) + H₂O
H2(g) + CO2(g)
CH4) + H₂O(g)
(1) CO(g) + H₂(g)
(2) CO(g) + H₂O(g) --->
(3) CO(g) + 3H2(g) --->
C) 64 min
CH4(g) + CO2(g)
AH-150 kJ
AH-41kJ
ΔΗ°==-206}J
A) 53 kJ
B) 116 kJ
C) -372 kJ
D)-116 kJ
18) When a strong acid is titrated with a weak base, the pH at the equivalence point is ALWAYS
A) <7
B) 7
9>7
D) <1
19) Which is the weakest intermolecular force among a group of molecules of comparable molar mass?
A) Dipole-dipole
B) London
C) Ionic bonding
D) Hydrogen bonding
20)
Given: N2(g) + 3 H₂(g) <-> 2 NH3(g)
At equilibrium at a certain temperature, the concentration of NH3(g), H₂(g) and N₂(g) are 0.490 M, 1.41 M
and 0.14 M, respectively. Calculate the value of Ke for this reaction.
A) 1.26
B) 3.02
C) 0.612
D) 1.96
21) Which one of these statements about strong base is true
A) Strong bases produce solutions with a higher pH than weak bases
B) Bases have only the OH group in their structure
C) Strong bases are very concentrated acids
D) Strong bases are 100% ionized in water
22) Consider the reaction represented by the equation: Fe³+ (aq) + SCN- (aq) → FeSCN²+ (aq)
6.00 M Fe³+ (aq) and 10.0 M SCN (aq) are mixed at a certain temperature and at equilibrium
the concentration of FeSCN2+ (aq) is 2.50 M.
A) 295x10-3
B) 303 x104
D) 95 x10³
C) 250x104
23) Consider the following reaction: 4 PCB(g)-> P4(g) + 6 Cl2(g)
If the initial concentration of PC13(g) is 3.0 M, and "x" is the equilibrium concentration of P4(g), what is the
correct equilibrium relation?
A) Kc = 6x7
B) Kc=6x7/(3.0-x)+
D) Kc=x7/(3.0-x)*
C) Kc = (x)(6x)/(3.0-4x)*
24) What is the density of NH3 at 5 atm pressure and a temperature of 35°C?
C) 0.980 g/L
B) 3.36 g/L
A) 16.6 g/L
25) What is the pH of 80.0 M aqueous solution of NH3 (ammonia). It's Kb = 1.8 x 10³.
B) 1.98
A)12.6
C) 0.778
D) 1.39 g/L
D) 0.0104
CAS
Expert Solution
Step 1
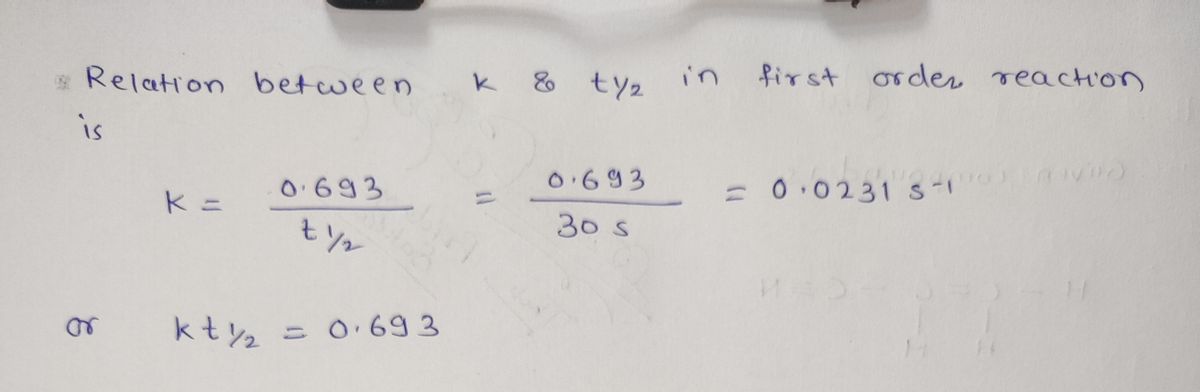
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY