1. Predict the major final product(s) for ANY FIVE of the following six problems. Assume one equivalent of each reagent unless indicated otherwise (parentheses indicate a catalytic amount, and ex = excess). If you have time at the end to do all six, I will use your best five. Count carbons carefully and keep track of all of them! a) Same product formed from left and right. Use the other one to verify your answer, if you have time at the end. H3C OH 1. HBr 2. KOC(CH3)3 CH₂=0 OPPh3
Reactive Intermediates
In chemistry, reactive intermediates are termed as short-lived, highly reactive atoms with high energy. They rapidly transform into stable particles during a chemical reaction. In specific cases, by means of matrix isolation and at low-temperature reactive intermediates can be isolated.
Hydride Shift
A hydride shift is a rearrangement of a hydrogen atom in a carbocation that occurs to make the molecule more stable. In organic chemistry, rearrangement of the carbocation is very easily seen. This rearrangement can be because of the movement of a carbocation to attain stability in the compound. Such structural reorganization movement is called a shift within molecules. After the shifting of carbocation over the different carbon then they form structural isomers of the previous existing molecule.
Vinylic Carbocation
A carbocation where the positive charge is on the alkene carbon is known as the vinyl carbocation or vinyl cation. The empirical formula for vinyl cation is C2H3+. In the vinyl carbocation, the positive charge is on the carbon atom with the double bond therefore it is sp hybridized. It is known to be a part of various reactions, for example, electrophilic addition of alkynes and solvolysis as well. It plays the role of a reactive intermediate in these reactions.
Cycloheptatrienyl Cation
It is an aromatic carbocation having a general formula, [C7 H7]+. It is also known as the aromatic tropylium ion. Its name is derived from the molecule tropine, which is a seven membered carbon atom ring. Cycloheptatriene or tropylidene was first synthesized from tropine.
Stability of Vinyl Carbocation
Carbocations are positively charged carbon atoms. It is also known as a carbonium ion.

We have given two reaction
a) In left side and
b) In right side
The reagents and starting material of both reactions are different but the product will be same , A (say).
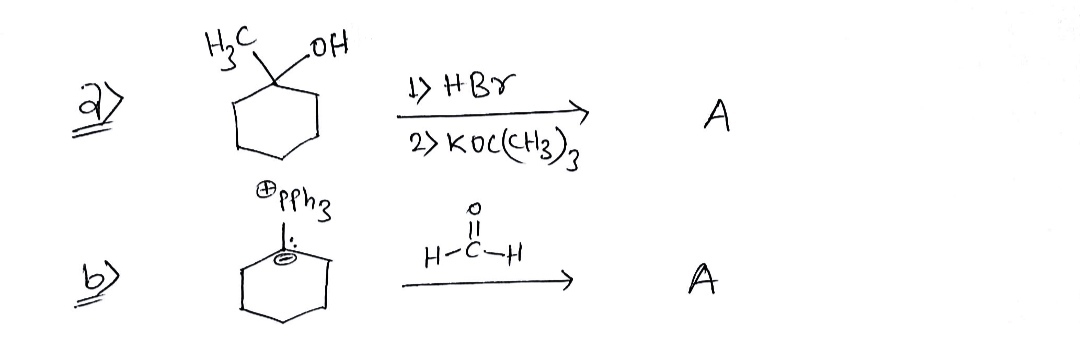
In reaction (a) a 3° alcohol is treated with HBr then KOC(CH3)3 which is a strong bulky base. These reaction conditions will lead to an elimination reaction forming a less substituted alkene.
In reaction (b) a phosphonium ylide is reacted with formaldehyde which lead to the formation of an alkene, this reaction is known as Wittig reaction
Step by step
Solved in 4 steps with 4 images









