. There is a very hot (600 °C) stream of oil exiting a reactor at 100 3. 1 kg/min that must be immediately quenched to 95 °C. One way to do this is to rapidly mix the oil with water available at 75 °F in a static mixer. The oil will cool, and the water will warm. After they are thermally equilibrated, the two can be separated by a simple decantation process (oil and water don't mix). Given that the heat capacity of oil is 2.56 kJ/kg-K, and that the heat capacity of water is 4.20 kJ/kg-K, what water flow is required? Oil at 95 °C 100 kg/min oil at 600 °C Mixer Mixture at 95 °C Water at 75 °F Water at 95 °C
. There is a very hot (600 °C) stream of oil exiting a reactor at 100 3. 1 kg/min that must be immediately quenched to 95 °C. One way to do this is to rapidly mix the oil with water available at 75 °F in a static mixer. The oil will cool, and the water will warm. After they are thermally equilibrated, the two can be separated by a simple decantation process (oil and water don't mix). Given that the heat capacity of oil is 2.56 kJ/kg-K, and that the heat capacity of water is 4.20 kJ/kg-K, what water flow is required? Oil at 95 °C 100 kg/min oil at 600 °C Mixer Mixture at 95 °C Water at 75 °F Water at 95 °C
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question
100%
3
ref book: Introduction to

Transcribed Image Text:3. г
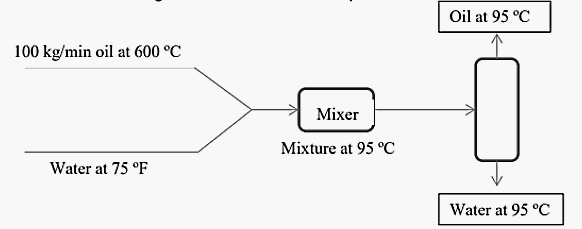
J. There is a very hot (600 °C) stream of oil exiting a reactor at 100
kg/min that must be immediately quenched to 95 °C. One way to do this is to
rapidly mix the oil with water available at 75 °F in a static mixer. The oil will
cool, and the water will warm. After they are thermally equilibrated, the two
can be separated by a simple decantation process (oil and water don't mix).
Given that the heat capacity of oil is 2.56 kJ/kg-K, and that the heat capacity
of water is 4.20 kJ/kg-K, what water flow is required?
Oil at 95 °C
100 kg/min oil at 600 °C
Mixer
Mixture at 95 °C
Water at 75 °F
Water at 95 °C
Expert Solution
Step 1
Initial oil temperature, Toil(1) = 600°C = 873 K
Initial water temperature, Twater(1) = 75°F = 297.039 K
Final mixture temperature, T2 = 95°C = 368 K
Flow rate of oil, moil = 100 kg/min
Specific heat capacity of oil, Coil = 2.56 kJ/kg.K
Specific heat capacity of water, Cwater = 4.20 kJ/kg.K
The schematic process diagram for the given process is:
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The