
ORGANIC CHEMISTRY-STUDY GDE./SOL.MAN.
6th Edition
ISBN: 9780072397475
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 9.14, Problem 28P
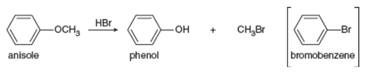
Explain why the treatment of anisole with
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Draw the products of the reaction shown below. Use wedge and dash bonds
to indicate stereochemistry. Ignore inorganic byproducts.
OSO4 (cat)
(CH3)3COOH
Select to Draw
ઘ
Calculate the reaction rate for selenious acid, H2SeO3, if 0.1150 M I-1 decreases to 0.0770 M in 12.0 minutes.
H2SeO3(aq) + 6I-1(aq) + 4H+1(aq) ⟶ Se(s) + 2I3-1(aq) + 3H2O(l)
Problem 5-31
Which of the following objects are chiral?
(a) A basketball
(d) A golf club
(b) A fork
(c) A wine glass
(e) A spiral staircase
(f) A snowflake
Problem 5-32
Which of the following compounds are chiral? Draw them, and label the chirality centers.
(a) 2,4-Dimethylheptane
(b) 5-Ethyl-3,3-dimethylheptane
(c) cis-1,4-Dichlorocyclohexane
Problem 5-33
Draw chiral molecules that meet the following descriptions:
(a) A chloroalkane, C5H11Cl
(c) An alkene, C6H12
(b) An alcohol, C6H140
(d) An alkane, C8H18
Problem 5-36
Erythronolide B is the biological precursor of
erythromycin, a broad-spectrum antibiotic. How
H3C
CH3
many chirality centers does erythronolide B have?
OH
Identify them.
H3C
-CH3
OH
Erythronolide B
H3C.
H3C.
OH
OH
CH3
Chapter 9 Solutions
ORGANIC CHEMISTRY-STUDY GDE./SOL.MAN.
Ch. 9.1 - Problem 9.1 Label each ether and alcohol in...Ch. 9.3 - Give the IUPAC name for each compound.Ch. 9.3 - Problem 9.3 Give the structure corresponding to...Ch. 9.3 - Name each of the following ethers.Ch. 9.3 - Name each epoxide.
a. (two ways) b. c. (two...Ch. 9.6 - Problem 9.8 Draw the organic product of each...Ch. 9.6 - Prob. 9PCh. 9.6 - Problem 9.10 Draw the products of each reaction.
...Ch. 9.8 - Problem 9.11 Draw the products formed when each...Ch. 9.8 - Prob. 12P
Ch. 9.11 - Problem 9.18 Draw the products of each reaction,...Ch. 9.11 - Problem 9.19 What is the major product formed...Ch. 9.12 - Prob. 19PCh. 9.12 - Prob. 20PCh. 9.12 - Problem 9.22 Draw the organic products formed in...Ch. 9.12 - Problem 9.23 Draw two steps to convert into each...Ch. 9.13 - Prob. 23PCh. 9.13 - Problem 9.25 Draw the products of each reaction,...Ch. 9.13 - Draw the products formed when (S)-butan-2-ol is...Ch. 9.13 - Draw the product formed when (CH3)2CHOH is treated...Ch. 9.14 - What alkyl halides are formed when each ether is...Ch. 9.14 - Explain why the treatment of anisole with HBr...Ch. 9.15 - Name each thiol.
a. b.
Ch. 9.15 - Draw the product of each reaction. ac b.d.Ch. 9.15 - Give the IUPAC name for each sulfide.
a. b.
Ch. 9.15 - Draw the product of each reaction.
a. b.
Ch. 9.16 - Prob. 33PCh. 9.16 - The cis and trans isomers of 2, 3-dimethyloxirane...Ch. 9.16 - Problem 9.36 Draw the product of each...Ch. 9 - 9.37 Name each compound depicted in the...Ch. 9 - Answer each question using the ball-and-stick...Ch. 9 - Prob. 38PCh. 9 - 9.40 Give IUPAC name for each...Ch. 9 - Prob. 40PCh. 9 - Prob. 41PCh. 9 - 9.46 What alkenes are formed when each alcohol is...Ch. 9 - Prob. 46PCh. 9 - Prob. 51PCh. 9 - 9.57 Draw a stepwise, detailed mechanism for the...Ch. 9 - Prob. 53PCh. 9 - 9.59 Draw two different routes to each of the...Ch. 9 - Prob. 55PCh. 9 -
9.61 Draw the products formed when each ether is...Ch. 9 - 9.62 Draw a stepwise mechanism for each...Ch. 9 - Draw a stepwise, detailed mechanism for the...Ch. 9 - Prob. 59PCh. 9 - Draw the products of each reaction. a.c. b.d.Ch. 9 - Prob. 64PCh. 9 - Prob. 75P
Additional Science Textbook Solutions
Find more solutions based on key concepts
Label each statement about the polynucleotide ATGGCG as true or false. The polynucleotide has six nucleotides. ...
General, Organic, and Biological Chemistry - 4th edition
2. Define equilibrium population. Outline the conditions that must be met for a population to stay in genetic e...
Biology: Life on Earth (11th Edition)
Why is it unlikely that two neighboring water molecules would be arranged like this?
Campbell Biology (11th Edition)
To test your knowledge, discuss the following topics with a study partner or in writing ideally from memory. Th...
HUMAN ANATOMY
What process causes the Mediterranean intermediate Water MIW to become more dense than water in the adjacent At...
Applications and Investigations in Earth Science (9th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Problem 5-48 Assign R or S configurations to the chirality centers in ascorbic acid (vitamin C). OH H OH HO CH2OH Ascorbic acid O H Problem 5-49 Assign R or S stereochemistry to the chirality centers in the following Newman projections: H Cl H CH3 H3C. OH H3C (a) H H H3C (b) CH3 H Problem 5-52 Draw the meso form of each of the following molecules, and indicate the plane of symmetry in each: OH OH (a) CH3CHCH2CH2CHCH3 CH3 H3C. -OH (c) H3C CH3 (b) Problem 5-66 Assign R or S configurations to the chiral centers in cephalexin, trade-named Keflex, the most widely prescribed antibiotic in the United States. H2N H IHH S Cephalexin N. CH3 CO₂Harrow_forwardSteps and explanationn please.arrow_forwardSteps and explanationn please.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Alcohols, Ethers, and Epoxides: Crash Course Organic Chemistry #24; Author: Crash Course;https://www.youtube.com/watch?v=j04zMFwDeDU;License: Standard YouTube License, CC-BY