
Concept explainers
Azo dyes are organic dyes that are used for many applications, such as the coloring of fabrics. Many azo dyes are derivatives of the organic substance azobenzene, Cl2H10N2. A closely related substance is hydrazobenzene, Cl2H12N2. The Lewis structures of these two substances are
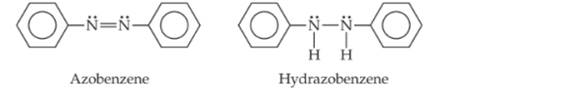
(Recall the shorthand notation used for benzene.)
a. What is the hybridization at the N atom in each of the substances?
b. How many unhybridized atomic orbitals are there on the N and the C atoms in each of the substances?
c. Predict the N-N-C angles in each of the substances.
d. Azobenzene is said to have greater delocalization of its p electrons than hydrazobenzene. Discuss this statement in light of your answers to (a) and (b).
e. All the atoms of azobenzene lie in one plane, whereas those of hydrazobenzene do not. Is this observation consistent with the statement in part (d)?
f. Azobenzene is an intense red-orange color, whereas hydrazobenzene is nearly colorless. Which molecule would be a better one to use in a solar energy conversion device? (See the "Chemistry Put to Work" box for more information about solar cells.)
Want to see the full answer?
Check out a sample textbook solution
Chapter 9 Solutions
EBK CHEMISTRY:CENTRAL SCIENCE
- Methylcyanoacrylate is the active ingredient in super glues. Its Lewis structure is (a) How many sigma bonds are in the molecule? (b) How many pi bonds are in the molecule? (c) What is the hybridization of the carbon atom bonded to nitrogen? (d) What is the hybridization of the carbon atom bonded to oxygen? (e) What is the hybridization of the double-bonded oxygen?arrow_forwardDo lone pairs about a central atom affect the hybridization of the central atom? If so, how?arrow_forwardIdentify the hybrid orbitals used by antimony in SbCl5 and in SbCl6, the ion formed from the reaction of SbCl5 and Cl. Explain your choices.arrow_forward
- 7.57 What observation about molecules compels us to consider the hybridization of atomic orbitals?arrow_forwardWhat hybrid orbitals would be expected for the central atom in each of the following molecules or ions?arrow_forwardIn propene CH3CH=CH2, the first carbon has sp3 hybrid orbitals and the second carbon has sp2 hybrid orbitals. These orbitals interact to make a bond. Why are these hybrid orbitals not orthogonal?arrow_forward
- The compound sketched below is acetylsalicylic acid, commonly known as aspirin. (a) What are the approximate values of the angles marked A, B, C, and D? (b) What hybrid orbitals are used by carbon atoms 1, 2, and 3ss?arrow_forwardIdentify the hybrid orbitals used by boron in BCl3 and in BCl4, the ion formed from the reaction of BCl3 and Cl. Explain your choices.arrow_forward7.59 What type of hybrid orbital is generated by combining the valence s orbital and all three valence p orbitals of an atom? How many hybrid orbitals result?arrow_forward
- Why is the concept of hybridization required in valence bond theory?arrow_forwardCompare and contrast the molecular orbital and ionic bonding descriptions of LiF.arrow_forwardThe structure of amphetamine, a stimulant, is shown below. (Replacing one H atom on the NH2, or amino, group with CH3 gives methamphetamine a particularly dangerous drug commonly known as speed.) (a) What are the hybrid orbitals used by the C atoms of the C6 ring. by the C atoms of the side chain, and by the N atom? (b) Give approximate values for the bond angles A, B, and C. (c) How many bonds and bonds are in the molerule? (d) Is the molecule polar or nonpolar? (e) Amphetamine reacts readily with a proton (H+) in aqueous solution. Where does this proton attach to the molecule? Explain how the electrostatic potential map predicts this site of protonation.arrow_forward
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781285199023Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781285199023Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning





