
Organic Chemistry Plus Masteringchemistry With Pearson Etext, Global Edition
9th Edition
ISBN: 9781292151229
Author: Wade, LeRoy G.
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 8, Problem 8.50SP
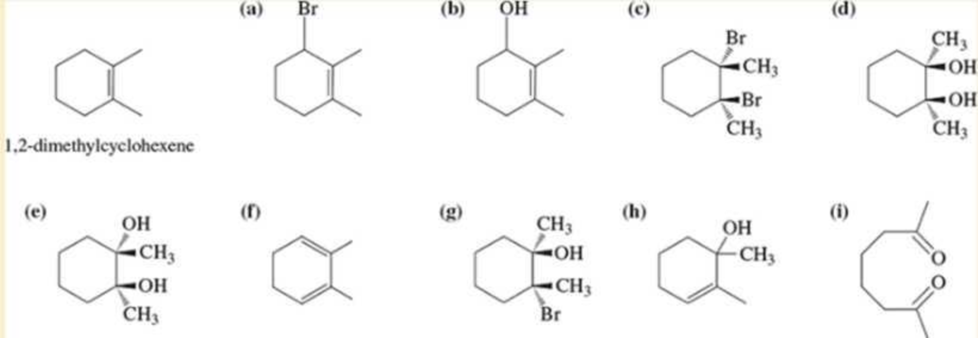
Using 1,2-dimethylcyclohexene as your starting material, show how you would synthesize the following compounds. (Once you have shown how to synthesize a compound, you may use it as the starting material in any later parts of this problem.) If a chiral product is shown, assume that it is part of a racemic mixture.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
The deuterated ethanol shown can be converted to an alkyl halide via a mesylate intermediate. Complete the mechanism, draw the final product (with nonbonding electrons) and select the correct absolute stereochemistry of the starting material and the final product.
Under certain reaction conditions, 2,3-dibromobutane reacts with two equivalents of base to give three products, each of which contains two new p bonds. Product A has two sp hybridized carbon atoms, product B has one sp hybridized carbon atom, and product C has none. What are the structures of A, B, and C?
Predict the major products of the following reaction. If it is possible, write all stereoisomers.
Chapter 8 Solutions
Organic Chemistry Plus Masteringchemistry With Pearson Etext, Global Edition
Ch. 8.3A - Predict the major products of the following...Ch. 8.3A - a. When 1 mole of buta-1,3-diene reacts with 1...Ch. 8.3B - Predict the major products of the following...Ch. 8.3B - Show how you would accomplish the following...Ch. 8.4B - Propose a mechanism to show how...Ch. 8.4B - Predict the products of the following hydration...Ch. 8.6 - a. Propose a mochansm fortho following reaction....Ch. 8.6 - Prob. 8.8PCh. 8.6 - Prob. 8.9PCh. 8.7A - Prob. 8.10P
Ch. 8.7A - Prob. 8.11PCh. 8.7C - Prob. 8.12PCh. 8.7C - Prob. 8.13PCh. 8.7C - a. When (Z)-3-methylhex-3-ene undergoes...Ch. 8.7C - Prob. 8.15PCh. 8.7C - Prob. 8.16PCh. 8.8B - Prob. 8.17PCh. 8.8B - Prob. 8.18PCh. 8.9 - Prob. 8.19PCh. 8.9 - Prob. 8.20PCh. 8.9 - Prob. 8.21PCh. 8.9 - Prob. 8.22PCh. 8.10 - Prob. 8.23PCh. 8.10 - Prob. 8.24PCh. 8.10 - Prob. 8.25PCh. 8.11A - Prob. 8.26PCh. 8.11B - Prob. 8.27PCh. 8.11B - Prob. 8.28PCh. 8.12 - Prob. 8.29PCh. 8.13 - a. Propose a mechanism for the conversion of...Ch. 8.13 - Magnesium monoperoxyphthalate (MMPP) epoxidizes...Ch. 8.13 - Predict the major products of the following...Ch. 8.13 - When 1,2-epoxycyclohexane (cyclohexene oxide) is...Ch. 8.14C - Predict the major products of the following...Ch. 8.14C - Prob. 8.35PCh. 8.15B - Prob. 8.36PCh. 8.15C - Predict the major products of the following...Ch. 8.16A - Prob. 8.38PCh. 8.16A - Prob. 8.39PCh. 8.16B - Prob. 8.40PCh. 8.16B - Prob. 8.41PCh. 8.16C - Prob. 8.42PCh. 8.17B - Prob. 8.43PCh. 8.17B - Prob. 8.44PCh. 8.17B - Show how you would synthesize each compound,...Ch. 8 - Prob. 8.46SPCh. 8 - Prob. 8.47SPCh. 8 - Give the products expected when the following...Ch. 8 - Show how you would make the following compounds...Ch. 8 - Using 1,2-dimethylcyclohexene as your starting...Ch. 8 - Show how you would synthesize each compound using...Ch. 8 - Prob. 8.52SPCh. 8 - Show how you might use olefin metathesis to...Ch. 8 - Prob. 8.54SPCh. 8 - Prob. 8.55SPCh. 8 - Propose mechanisms consistent with the following...Ch. 8 - Prob. 8.57SPCh. 8 - Prob. 8.58SPCh. 8 - Draw a reaction-energy diagram for the propagation...Ch. 8 - Prob. 8.60SPCh. 8 - Prob. 8.61SPCh. 8 - Prob. 8.62SPCh. 8 - Prob. 8.63SPCh. 8 - Prob. 8.64SPCh. 8 - Prob. 8.65SPCh. 8 - Prob. 8.66SPCh. 8 - Prob. 8.67SPCh. 8 - Prob. 8.68SPCh. 8 - Prob. 8.69SPCh. 8 - Prob. 8.70SPCh. 8 - Prob. 8.71SPCh. 8 - Prob. 8.72SPCh. 8 - Prob. 8.73SPCh. 8 - Prob. 8.74SPCh. 8 - Prob. 8.75SPCh. 8 - Prob. 8.76SPCh. 8 - Prob. 8.77SPCh. 8 - Prob. 8.78SPCh. 8 - Prob. 8.79SP
Additional Science Textbook Solutions
Find more solutions based on key concepts
Give the IUPAC name for each compound.
Organic Chemistry
Why do scientists think that all forms of life on earth have a common origin?
Genetics: From Genes to Genomes
Why are mutants used as test organisms in the Ames test?
Laboratory Experiments in Microbiology (12th Edition) (What's New in Microbiology)
Choose the best answer to each of the following. Explain your reasoning. If Earth were twice as far as it actua...
Cosmic Perspective Fundamentals
An obese 55-year-old woman consults her physician about minor chest pains during exercise. Explain the physicia...
Biology: Life on Earth with Physiology (11th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Nonconjugated , -unsaturated ketones, such as 3-cyclohexenone, are in an acid-catalyzed equilibrium with their conjugated , -unsaturated isomers. Propose a mechanism for this isomerization.arrow_forward(a) When cis-1-bromo-2-methylcyclohexane undergoes an E2 reaction, two products (cycloalkenes) are formed. What are these two cycloalkenes, and which would you expect to be the major product? Write conformational structures showing how each is formed. (b) When rans-1-bromo-2-methylcyclohexane reacts in an E2 reaction, only one cyclo- alkene is formed. What is this product? Write conformational structures showing why it is the only product.arrow_forwardNon-conjugated β,γ-unsaturated ketones, such as 3-cyclohexenone, as in acid-catalysed equilibrium with their α,β-unsaturated isomers. The mechanism has several intermediates. Draw the structure of the second reaction intermediate in the conversion of 3-cyclohexenone to 2-cyclohexenone. This intermediate is a neutral compound.arrow_forward
- The compound 3,4-dimethyl-hexan-3-ol of 3R, 4S configuration is treated with a concentrated HBr solution at room temperature. A mixture of two stereoisomers is obtained.If the reaction mixture above is heated, the appearance of several other compounds is observed. 1) Draw the different compounds obtained using the wedge-flywheel representation. 2) What is the majority product? Explain 3) Propose a modification of the experimental conditions in order to obtain the exclusive formation of these compounds obtained after heatingarrow_forwardThe alkene shown undergoes bromination. H (a) Draw the product(s) of bromination of this compound, including all expected stereoisomers (if any). Use wedge-and-dash bonds to designate the stereochemistry at any chirality centers, and make sure to draw an explicit hydrogen if a chirality center has one. (b) Characterize the starting alkene as having the E or Z configuration. (c) characterize the product(s). (a) H Br₂ Draw the product(s) of bromination. Br H Brarrow_forwardThe alkene shown undergoes bromination. (a) Draw the product(s) of bromination of this compound, including all expected stereoisomers (if any). Use wedge‑and‑dash bonds to designate the stereochemistry at any chirality centers, and make sure to draw an explicit hydrogen if a chirality center has one. (b) Characterize the starting alkene as having the E or Z configuration. (c) characterize the product(s).arrow_forward
- Compound A has molecular formula C7H15B.. Treatment of compound A with sodium ethoxide yields only one elimination product (compound B) and no substitution products. When compound B is treated with dilute sulfuric acid, compound C is obtained, which has molecular formula C7H160. Draw the structures of compounds A, B, and C.arrow_forwardWhen trans-2-chloro-1-cyclohexanol is treated with a base, cyclohexene oxide is the product. However, when cis-2-chloro-1-cyclohexanol is treated with a base, the product is cyclohexanone. Write the mechanism for each of the two reactions.arrow_forwardCompound A Br₂, H₂O Compound B (C8H15BrO) + enantiomer CH₂O O Compound C + enantiomer Draw the structure of Compound B (watch out for stereochemistry), and mechanisms for its formation from Compound A, and its conversion to Compound C.arrow_forward
- (a) Show how you would synthesize the pure (R) enantiomer of 2-butyl methyl sulfide, starting with pure (R)-butan-2-oland any reagents you need.(b) Show how you would synthesize the pure (S) enantiomer of the product, still starting with (R)-butan-2-ol and anyreagents you need.arrow_forwardA graduate student was studying enzymatic reductions of cyclohexanones when she encountered some interesting chemistry. When she used an enzyme and NADPH to reduce the following ketone, she was surprised to find that the product was optically active. She carefully repurified the product so that no enzyme, NADPH, or other contaminants were present. Still, the product was optically active. Does the product have any asymmetric carbon atoms or other stereocenters?arrow_forwardThe bicyclo [3.1.0] hexane ring system, highlighted in compound 3, is found in several natural products, including sabinene, a compound partially responsible for the flavor of ground black pepper. One method for preparing this ring system involves the conversion of compound 1 to compound 2, as shown below. Draw the structure of compound 2 and provide a reasonable mechanism for its formation. Add any remaining curved arrows to complete step one of the mechanism, and modify the given drawing as needed to show the intermediate that is formed in this step.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

How to Design a Total Synthesis; Author: Chemistry Unleashed;https://www.youtube.com/watch?v=9jRfAJJO7mM;License: Standard YouTube License, CC-BY