
Interpretation: The mechanism for the given substrate should be determined under given conditions.
Concept Introduction:
Structure of the substrate plays major role in the reactivity of
The
Intramolecular
Leaving group: It is a fragment that leaves substrate with a pair of electrons via heterolytic bond cleavage.
Nucleophile: Donates pair of electrons to positively charged substrate resulting in the formation of
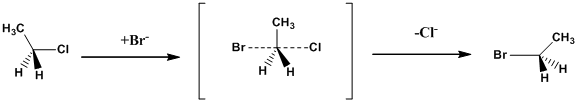
Example:
Want to see the full answer?
Check out a sample textbook solution
Chapter 7 Solutions
Organic Chemistry, Binder Ready Version
- Steps and explanation.arrow_forwardProvide steps and explanation please.arrow_forwardDraw a structural formula for the major product of the acid-base reaction shown. H 0 N + HCI (1 mole) CH3 N' (1 mole) CH3 You do not have to consider stereochemistry. ● • Do not include counter-ions, e.g., Na+, I, in your answer. . In those cases in which there are two reactants, draw only the product from 989 CH3 344 ? [Farrow_forward
- Assign these protonarrow_forwardCould you please solve the first problem in this way and present it similarly but color-coded or step by step so I can understand it better? Thank you!arrow_forwardCould you please solve the first problem in this way and present it similarly but color-coded or step by step so I can understand it better? Thank you!arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





