
(a)
Interpretation: The carbocation intermediate and the type of carbocation present in the given substrates should be identified.
Concept introduction:
Carbocation intermediate: It is a fragment from the molecule in which the carbon atom bears a positive charge on it. The stability of the carbocation present in a molecule depends on the neighboring atom present in the molecule with respect to its resonance effects.
Resonance: The delocalization of pi electrons or lone pair of electrons present in p orbital within a molecule. The molecule is said to be more stable if it is resonance stabilized.
The order of the stability of carbocation,
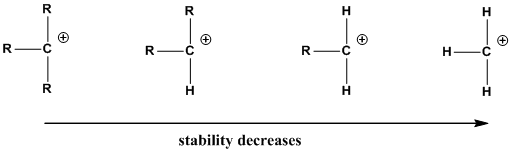
(b)
Interpretation: The carbocation intermediate and the type of carbocation present in the given substrates should be identified.
Concept introduction:
Carbocation intermediate: It is a fragment from the molecule in which the carbon atom bears a positive charge on it. The stability of the carbocation present in a molecule depends on the neighboring atom present in the molecule with respect to its resonance effects.
Resonance: The delocalization of pi electrons or lone pair of electrons present in p orbital within a molecule. The molecule is said to be more stable if it is resonance stabilized.
The order of the stability of carbocation,

(c)
Interpretation: The carbocation intermediate and the type of carbocation present in the given substrates should be identified.
Concept introduction:
Carbocation intermediate: It is a fragment from the molecule in which the carbon atom bears a positive charge on it. The stability of the carbocation present in a molecule depends on the neighboring atom present in the molecule with respect to its resonance effects.
Resonance: The delocalization of pi electrons or lone pair of electrons present in p orbital within a molecule. The molecule is said to be more stable if it is resonance stabilized.
The order of the stability of carbocation,

(d)
Interpretation: The carbocation intermediate and the type of carbocation present in the given substrates should be identified.
Concept introduction:
Carbocation intermediate: It is a fragment from the molecule in which the carbon atom bears a positive charge on it. The stability of the carbocation present in a molecule depends on the neighboring atom present in the molecule with respect to its resonance effects.
Resonance: The delocalization of pi electrons or lone pair of electrons present in p orbital within a molecule. The molecule is said to be more stable if it is resonance stabilized.
The order of the stability of carbocation,

Want to see the full answer?
Check out a sample textbook solution
Chapter 7 Solutions
Organic Chemistry, Binder Ready Version
- true or false The equilibrium constant for this reaction is 0.20. N2O4(g) ⇔ 2NO2(g) Based on the above, the equilibrium constant for the following reaction is 5. 4NO2(g) ⇔ 2N2O4(g)arrow_forwardtrue or false The equilibrium constant for this reaction is 0.20. N2O4(g) ⇔ 2NO2(g) Based on the above, the equilibrium constant for the following reaction is 0.4. 2N2O4(g) ⇔ 4NO2(g)arrow_forwardtrue or false Using the following equilibrium, if heat is added the equilibrium will shift toward the reactants. N2(g) + 3H2(g) ⇔ 2NH3(g) + heatarrow_forward
- True or False Using the following equilibrium, if heat is added the equilibrium will shift toward the products. N2O4(g) + heat ⇔ 2NO2(g)arrow_forwardtrue or false Using the following equilibrium, if solid carbon is added the equilibrium will shift toward the products. C(s) + CO2(g) ⇔ 2CO(g)arrow_forwardProvide the complete mechanism for the reaction below. You must include appropriate arrows,intermediates, and formal charges. Please also provide a reason to explain why the 1,4-adduct is preferred over the 1,3-adduct.arrow_forward
- Which of the following pairs are resonance structures of one another? I. III. || III IV + II. :0: n P !༠ IV. EN: Narrow_forwardPredict the major organic product(s) and byproducts (either organic or inorganic) for thefollowing reactions.arrow_forwardA 8.25 g sample of aluminum at 55°C released 2500 J of heat. The specific heat of aluminum is 0.900 J/g°C. The density of aluminum is 2.70 g/mL. Calculate the final temperature of the aluminum sample in °C.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





