
EP BASIC CHEMISTRY-STANDALONE ACCESS
6th Edition
ISBN: 9780134999890
Author: Timberlake
Publisher: PEARSON CO
expand_more
expand_more
format_list_bulleted
Question
Chapter 6, Problem 61UTC
Interpretation Introduction
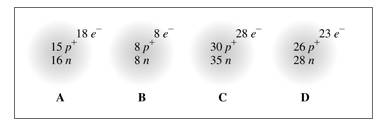
To identify: The given atoms or ions.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Examine the following hypothetical ion
WZY (Z in the central atom)
Each atom has the following valence (electronegativities in brackets)
W=5 (3.1)
Z=4 (2.2)
Y=6 (2.4)
There are three possible lewis structures for this ion. Draw all three and use formal
charges to identify the best.
7. What type of product is formed when acids are added to some ionic compounds?
Stronger acids
Weaker acids
Gas
Solids
GET
New version available! (3.0.220)
(2.6) How many protons and electrons does phosphide ion (P3-) contains in its structure?
protons
[Choose ]
electrons
[Choose ]
4
Previous
>
Chapter 6 Solutions
EP BASIC CHEMISTRY-STANDALONE ACCESS
Ch. 6.1 - State the number of electrons that must be lost by...Ch. 6.1 - State the number of electrons that must be gained...Ch. 6.1 - State the number of electrons lost or gained when...Ch. 6.1 - Prob. 4PPCh. 6.1 - Prob. 5PPCh. 6.1 - Write the symbols for the ions with the following...Ch. 6.1 - State the number of protons and electrons in each...Ch. 6.1 - Prob. 8PPCh. 6.1 - Prob. 9PPCh. 6.1 - Write the symbol for the ion of each of the...
Ch. 6.1 - Write the names for each of the following ions:...Ch. 6.1 - Write the names for each of the following ions:...Ch. 6.1 - Prob. 13PPCh. 6.1 - Prob. 14PPCh. 6.2 - Which of the following pairs of elements are...Ch. 6.2 - Which of the following pairs of elements are...Ch. 6.2 - Prob. 17PPCh. 6.2 - Write the correct ionic formula for the compound...Ch. 6.2 - Prob. 19PPCh. 6.2 - Prob. 20PPCh. 6.3 - Prob. 21PPCh. 6.3 - Prob. 22PPCh. 6.3 - Write the name for each of the following ions...Ch. 6.3 - Prob. 24PPCh. 6.3 - Prob. 25PPCh. 6.3 - Prob. 26PPCh. 6.3 - Prob. 27PPCh. 6.3 - Prob. 28PPCh. 6.3 - Write the formula for each of the following ionic...Ch. 6.3 - Prob. 30PPCh. 6.3 - Prob. 31PPCh. 6.3 - Prob. 32PPCh. 6.3 - The following compounds contain ions that are...Ch. 6.3 - Prob. 34PPCh. 6.4 - Prob. 35PPCh. 6.4 - Prob. 36PPCh. 6.4 - Prob. 37PPCh. 6.4 - Prob. 38PPCh. 6.4 - Prob. 39PPCh. 6.4 - Prob. 40PPCh. 6.4 - Prob. 41PPCh. 6.4 - Prob. 42PPCh. 6.4 - Prob. 43PPCh. 6.4 - Prob. 44PPCh. 6.4 - Name each of the following ionic compounds:...Ch. 6.4 - Prob. 46PPCh. 6.5 - Name each of the following molecular compounds:...Ch. 6.5 - Name each of the following molecular compounds:...Ch. 6.5 - Name each of the following molecular compounds:...Ch. 6.5 - Prob. 50PPCh. 6.5 - Prob. 51PPCh. 6.5 - Prob. 52PPCh. 6.5 - Prob. 53PPCh. 6.5 - Prob. 54PPCh. 6.5 - Name each of the following ionic or molecular...Ch. 6.5 - Prob. 56PPCh. 6.5 - Prob. 57PPCh. 6.5 - Prob. 58PPCh. 6 - The chapter sections to review are shown in...Ch. 6 - Prob. 60UTCCh. 6 - Prob. 61UTCCh. 6 - Prob. 62UTCCh. 6 - The chapter sections to review are shown in...Ch. 6 - The chapter sections to review are shown in...Ch. 6 - Prob. 65UTCCh. 6 - Prob. 66UTCCh. 6 - Prob. 67APPCh. 6 - Prob. 68APPCh. 6 - Prob. 69APPCh. 6 - Prob. 70APPCh. 6 - One of the ions of tin is tin(IV). (6.1, 6.2, 6.3,...Ch. 6 - Prob. 72APPCh. 6 - Prob. 73APPCh. 6 - Prob. 74APPCh. 6 - Prob. 75APPCh. 6 - Prob. 76APPCh. 6 - Write the formula for each of the following...Ch. 6 - Write the formula for each of the following...Ch. 6 - Classify each of the following as ionic or...Ch. 6 - Prob. 80APPCh. 6 - Prob. 81APPCh. 6 - Prob. 82APPCh. 6 - Prob. 83CPCh. 6 - Prob. 84CPCh. 6 - Prob. 85CPCh. 6 - Prob. 86CPCh. 6 - Prob. 87CPCh. 6 - The following problems are related to the topics...
Knowledge Booster
Similar questions
- A metal ion with 2+ charge has 23 electrons and forms a compound with a halogen ion that contains 17 protons. (3)arrow_forward(10.6) Classify each of the following bonds as nonpolar covalent, polar covalent, or ionic bond. bond between two fluorine atoms bond between sodium and nitrogen bond between carbon and fluorine [Choose ] [Choose ] ionic polar covalent nonpolar covalent [Choose ]arrow_forward11.Starting from the number of moles (7.2)How many K+ ions are there? a)5.06 x 10 to the 24 K+ ions b)16.8 x 10 to the 23 K'+ ions c)1.2 x 10 to the 24 K+ ions d)8.66 x 10 to the 24 K+ ions e)1.01 x 10 to the 25 K+ ionsarrow_forward
- 7. 8. 9. 10 11 12 13 F 14 P 15 P16 17 18 19 20 6.00 g of a certain Compound X, known to be made of carbon, hydrogen and perhaps oxygen, and to have a molecular molar mass of 26. g/mol, is burned completely in excess oxygen, and the mass of the products carefully measured: product mass carbon dioxide 20.31 g water 4.16 g Use this information to find the molecular formula of X. Submit Assignn ontilyue Chparrow_forwardquestion 9arrow_forward9. Write the correct formulas for the following compounds and state if they are molecular or ionic. 2. 1. 3. + 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. Compound sodium sulfide iron(III) sulfite nitrogen trioxide lead(II) iodide sodium carbonate potassium perchlorate diphosphorus pentoxide methane butane calcium bromide ethane propane dichlorine heptoxide Formula Ionic/Moleculararrow_forward
- 6. From the Periodic table, choose 2 metals from the Alkaline petal series, 2 metals from the transition metalies and 1 non-metal from the 6A group and write the noble gas configuration of each one of them. ( 7. Write the Nobie gas configuration (NGC) of the following lons. Write the symbol of the ion and the charge of the ion. (10 points). a Sodium ion Eve s b. Chloride lon c. Oxide ion d. Magnesium ion e. Sulfide ion 3. Pick the frequency of your most favorite radio station and calculate the wavelength (lambda) of the wavelength. Using this lambda value, calculate the Energy of the wave. Show your work, and include the units in your answer, wherever applicable. Show the steps on how you solved this problem.arrow_forward(3.6)What is the formula of the compound that forms between Pb4+ ion and sulfite ion? Pb₂SO3 O Pb2(SO3)4 O Pb(SO3)2 O Pb4(SO3)2 << Previousarrow_forwardProblem 7.48 I Review I Constant Calculate the number of moles in 3.43 g of each of the following: Part C Cr(ОН)з Express your answer with the appropriate units. HA Value Units n = Submit Previous Answers Request Answer X Incorrect; Try Again; 4 attempts remaining « Previousarrow_forward
- (3.6)Write the systematic names of compounds with lowercase letters except Roman Numerals. Roman number should be written with capital letters (I, II, III, IV, etc.). There should be no space between the end of the name of the metal and the parentheses with the Roman numeral. Example: CuO copper(II) oxide H₂CrO4(aq) SO3 acer H₂S(aq) FeN Sn3(PO3)2 1080arrow_forwardWhat mass (in grams) of magnesium oxide can be produced from igniting 1.5 g of Mg in Oxygen? (MM of O-16; Mg-24.3)arrow_forwardExample 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. Part 2: Cation Na+ Gat Cat AI+ Na+ Nat Na+ K+ AI+ NH4 Gat 1. Explain, in terms of Anion CI O PO CI P SO4 OH- P- OH P OH Chemical Formula The charge for element X is NaCl Ga₂(03) CA3(PO4)2 AICI Na3P Na₂(SO4) NaOH KP Al(OH)3 (NH4)3PO4 Ga(OH)3 ons, what a positive ion indicates. A positive ion indicates that it has more electrons. 2. Explain, in terms of electrons, what a negative ion indicates. A negative ion indicates that it gained one of more electrons. 3. Why do some ionic compounds contain a roman numeral? Name Sodium Chloride Gallium Oxygen Calcium Phosphate Aluminium Chlorine Sodium Phosphous Sodium Sulfate Sodium Hydroxide Keplerium Aluminium Hydroxide Ammonium Phosphorus Gallium Hydroxide 4. If element X combines with oxygen to form an ionic compound X₂O, what is the charge of element X? What Group does X belong to on the Periodic Table?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning