
ORGANIC CHEMISTRY-PACKAGE >CUSTOM<
10th Edition
ISBN: 9781260028355
Author: Carey
Publisher: MCG CUSTOM
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 6, Problem 21P
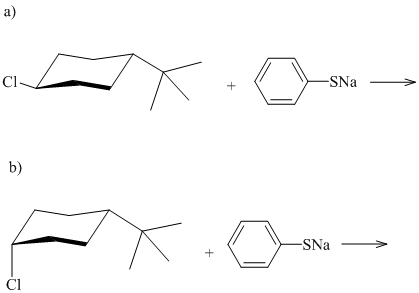
Both of the following reactions involve nucleophilic substitution. The product of reaction
(a) is an isomer of the product of reaction (b). What kind of isomer? By what mechanism does nucleophilic substitution occur? Write the structural formula of the product of each reaction.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
From the given compound, choose the proton that best fits each given description.
a
CH2
CH 2
Cl
b
с
CH2
F
Most shielded:
(Choose one)
Least shielded:
(Choose one)
Highest chemical shift:
(Choose one)
Lowest chemical shift:
(Choose one)
×
Consider this molecule:
How many H atoms are in this molecule?
How many different signals could be found in its 1H NMR spectrum?
Note: A multiplet is considered one signal.
For each of the given mass spectrum data, identify whether the compound contains chlorine, bromine, or neither.
Compound
m/z of M* peak
m/z of M
+ 2 peak
ratio of M+ : M
+ 2 peak
Which element is present?
A
122
no M
+ 2 peak
not applicable
(Choose one)
B
78
80
3:1
(Choose one)
C
227
229
1:1
(Choose one)
Chapter 6 Solutions
ORGANIC CHEMISTRY-PACKAGE >CUSTOM<
Ch. 6.1 - Prob. 1PCh. 6.2 - 1-Bromo-3-chloropropane reacts with one molar...Ch. 6.3 - Prob. 3PCh. 6.3 - The Fischer projection for (+)-2-bromooctane is...Ch. 6.3 - Would you expect the 2-octanol formed by SN2...Ch. 6.3 - Prob. 6PCh. 6.4 - Prob. 7PCh. 6.4 - The first step in the synthesis of the...Ch. 6.6 - Prob. 9PCh. 6.6 - Prob. 10P
Ch. 6.7 - Prob. 11PCh. 6.8 - Prob. 12PCh. 6.9 - Diethyl ether (CH3CH2OCH2CH3) has a dielectric...Ch. 6.9 - Unlike protic solvent which solvate from complexes...Ch. 6.10 - Prob. 15PCh. 6.10 - Prob. 16PCh. 6.10 - The hydrolysis of sulfonate of 2-octanol is...Ch. 6.11 - Prob. 18PCh. 6 - Prob. 19PCh. 6 - Prob. 20PCh. 6 - Both of the following reactions involve...Ch. 6 - Prob. 22PCh. 6 - Prob. 23PCh. 6 - Sodium nitrite (NaNO2) reacted with 2-iodooctane...Ch. 6 - Prob. 25PCh. 6 - Prob. 26PCh. 6 - Prob. 27PCh. 6 - The reaction of 2,2-dimethyl-1-propanol with HBr...Ch. 6 - If the temperature is not kept below 25oC during...Ch. 6 - The reaction of cyclopentyl bromide with sodium...Ch. 6 - Prob. 31PCh. 6 - Prob. 32PCh. 6 - Write an equation, clearly showing the...Ch. 6 - Prob. 34PCh. 6 - Based on what we know about nucleophiles and...Ch. 6 - Prob. 36PCh. 6 - Prob. 37PCh. 6 - Prob. 38PCh. 6 - Prob. 39PCh. 6 - Prob. 40PCh. 6 - Prob. 41DSPCh. 6 - Prob. 42DSPCh. 6 - Prob. 43DSPCh. 6 - Prob. 44DSPCh. 6 - Prob. 45DSPCh. 6 - Prob. 46DSP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Don't used hand raiting and don't used Ai solutionarrow_forward2' P17E.6 The oxidation of NO to NO 2 2 NO(g) + O2(g) → 2NO2(g), proceeds by the following mechanism: NO + NO → N₂O₂ k₁ N2O2 NO NO K = N2O2 + O2 → NO2 + NO₂ Ко Verify that application of the steady-state approximation to the intermediate N2O2 results in the rate law d[NO₂] _ 2kk₁[NO][O₂] = dt k+k₁₂[O₂]arrow_forwardPLEASE ANSWER BOTH i) and ii) !!!!arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


Macroscale and Microscale Organic Experiments
Chemistry
ISBN:9781305577190
Author:Kenneth L. Williamson, Katherine M. Masters
Publisher:Brooks Cole

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Characteristic Reactions of Benzene and Phenols; Author: Linda Hanson;https://www.youtube.com/watch?v=tjEqEjDd87E;License: Standard YouTube License, CC-BY
An Overview of Aldehydes and Ketones: Crash Course Organic Chemistry #27; Author: Crash Course;https://www.youtube.com/watch?v=-fBPX-4kFlw;License: Standard Youtube License