
EP CHEMISTRY:CENTRAL..-MOD.MASTERING
14th Edition
ISBN: 9780136781509
Author: Brown
Publisher: PEARSON CO
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 3, Problem 76E
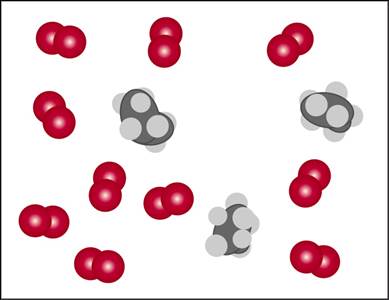
Consider the mixture of propane, C3H8, and O2 shown here.
- Write a balanced equation for the combustion reaction that occurs between propane and oxygen.
- Which reactant is the limiting reactant?
- How many molecules of CO2, H2O, C3H8, and O2will be present if the reaction goes to completion?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Chapter 3 Solutions
EP CHEMISTRY:CENTRAL..-MOD.MASTERING
Ch. 3.1 - In the following diagram, the white spheres...Ch. 3.1 - In the following digram, the white spheres...Ch. 3.1 - The unbalanced equation for the reaction between...Ch. 3.1 - Balance these equations by providing the missing...Ch. 3.2 - Which of the following reactions is the balanced...Ch. 3.2 - Write a balanced equation for (a) solid...Ch. 3.2 - Write the balanced equation for the reaction that...Ch. 3.2 - Prob. 3.4.2PECh. 3.3 - Which of the following is the correct formula...Ch. 3.3 - Prob. 3.5.2PE
Ch. 3.3 - What is the percentage of nitrogen, by mass, in...Ch. 3.3 - Calculate the percentage of potassium by mass in...Ch. 3.4 - Which of the following samples contains the fewest...Ch. 3.4 - Without using a calculator, arrange these samples...Ch. 3.4 -
How many sulfur are in (a) 0.45 mol BaSo4 and (b)...Ch. 3.4 -
How many oxygen atoms are in (a) 0.25 mol...Ch. 3.4 - A sample of an ionic compound containing iron and...Ch. 3.4 - Calculate the molar mass of Ca(NO3)2Ch. 3.4 - A 508-g sample of sodium bicarbonate (NaHCO3)...Ch. 3.4 - Prob. 3.10.2PECh. 3.4 - What is the mass, in grams, of 6.33 mol of NaHC03...Ch. 3.4 - What is the mass, in grams, of (a) 0.50 mol of...Ch. 3.4 - How many chlorine atoms are in 12.2 g of CCL4? a....Ch. 3.4 -
a. How many nitric acid molecules are in 4.20 g...Ch. 3.5 - A 2.144-g sample of phosgene, a compound used as a...Ch. 3.5 - A 5.325-g sample of methyl benzoate, a compound...Ch. 3.5 -
Cyclohexane a commonly used organic solvent, is...Ch. 3.5 - Prob. 3.14.2PECh. 3.5 -
The compound dioxane, which is used as a solvent...Ch. 3.5 -
a. Caproic acid, responsible for the odor of...Ch. 3.6 - Sodium hydroxide reacts with carbon dioxide to...Ch. 3.6 - Decomposition of KCIO3 is sometimes used to...Ch. 3.6 - Propane, C3 H8 (Figure 3.8), is a common fuel used...Ch. 3.6 -
Methanol, CH3OH, reacts with oxygen from air in a...Ch. 3.7 - When 24 mol of methanol and 15 mol of oxygen...Ch. 3.7 - a. When 1.50 mol of Al and 3.00 mol of Cl2 combine...Ch. 3.7 - Molten gallium reacts with arsenic to form the...Ch. 3.7 -
When a 2.00-g strip of zinc metal is placed in...Ch. 3.7 - If 3.00 g of titanium metal is reacted with 6.00 g...Ch. 3.7 - Imagine you are working on ways to improve the...Ch. 3 - The reaction between reactant A (blue spheres) and...Ch. 3 - The following diagram shows the combination...Ch. 3 -
3.3 The following diagram represents the...Ch. 3 -
3.4 The following diagram represents the...Ch. 3 - Glycine, an amino acid used by organisms to make...Ch. 3 - The following diagram represents a...Ch. 3 -
3.7 Nitrogen (N2) and hydrogen (H2) react to form...Ch. 3 -
3.8 Nitrogen monoxide and oxygen react to form...Ch. 3 - Write "true" or "false" for each statement. a. We...Ch. 3 - A key step in balancing chemical equations is...Ch. 3 - Balance the following equations: a. CO(g)...Ch. 3 - Balance the following equations 3. Li(s) + N2(g)...Ch. 3 -
3.13 Balance the following equations:
A14C3(s) +...Ch. 3 -
3.14 Balance the following equations:
a. Ca3P2(s)...Ch. 3 -
3.15 Write balanced chemical equations...Ch. 3 - Prob. 16ECh. 3 - Prob. 17ECh. 3 -
318
a. When a compound containing C, H, and O is...Ch. 3 - Write a balanced chemical equation for the...Ch. 3 -
3.20 Write a balanced chemical equation for the...Ch. 3 - Balance the following equations and indicate...Ch. 3 - Balance the following equations and indicate...Ch. 3 - Determine the formula weights of each of the...Ch. 3 - 3.24 Determine the formula weights of each of the...Ch. 3 - Calculate the percentage by mass of oxygen in the...Ch. 3 - Calculate the percentage by mass of the indicated...Ch. 3 - Based on the following structural formulas,...Ch. 3 - Calculate the percentage of carbon by mass In each...Ch. 3 - Write "true' or 'Yalse' for each statement a- A...Ch. 3 - a. What is the mass, in grams, of one mole of 12C?...Ch. 3 - Without doing any detailed calculations (but using...Ch. 3 - Without doing any detailed calculations {but using...Ch. 3 - What is the mass, m Iqlograms, of an Avogadro“s...Ch. 3 - If Avogadro’s number of pennies is divided equally...Ch. 3 - Calculate the following quantities: a. mass, in...Ch. 3 - Calculate the following quantities: a. mass, in...Ch. 3 - a. What is the mass, in grams, of 2.50 x 10-3 mol...Ch. 3 - a. What is the mass, in grams, of 1.223 mol of...Ch. 3 -
339 The molecular formula of allicin, the...Ch. 3 -
3.40 The molecular formula of aspartame, the...Ch. 3 -
3.41 A sample of glucose, C6H12O6, contains 1.250...Ch. 3 - A sample of the male sex hormone testosterone,...Ch. 3 -
343 The allowable concentration level of vinyl...Ch. 3 - At least 25 g oftetrahydrocannabinol (THC), the...Ch. 3 - Give the empirical formula of each of the...Ch. 3 - Determine the empirical formula of each of the...Ch. 3 - Determine the empirical formulas of the compounds...Ch. 3 - Determine the empirical formulas of the compounds...Ch. 3 -
3.49 A compound whose empirical formula is XF3...Ch. 3 - The compound XCL4contains 75.0% CI by mass What is...Ch. 3 -
3.51 What is the molecular formula of each of the...Ch. 3 - What is the molecular formula of each of the...Ch. 3 - Determine the empirical and molecular formulas of...Ch. 3 - Determine the empirical and molecular formulas of...Ch. 3 - a. Combustion analysis of toluene, a common...Ch. 3 - a. The characteristic odor of pineapple is due to...Ch. 3 -
3.57 Valproic acid, used to treat seizures and...Ch. 3 - Propenoic acid, C3H4O2, is a reactive organic...Ch. 3 -
3.59 Washing soda, a compound used to prepare...Ch. 3 -
3.60 Epsom salts, a strong laxative used in...Ch. 3 - f 3461 Hydrofluoric acid, HF(aq), cannot be stored...Ch. 3 - The reaction between potassium superoxide, KO2,...Ch. 3 -
3,63 Several brands of antacids use Al(OH)3 to...Ch. 3 -
3.64 An iron ore sample contains Fe2O3 together...Ch. 3 - Aluminum sulfides reacts with water to form...Ch. 3 - Calcium hydride reacts with water to form calcium...Ch. 3 -
3.67 Automotive air bags infilate when sodium...Ch. 3 - The complete combustion of octane, Cngs, a...Ch. 3 -
3.69 A piece of aluminum foil 1.00 cm2 and...Ch. 3 - Detonation of nitroglycerin proceeds as follows:...Ch. 3 -
3.71 The combustion of one mole of liquid...Ch. 3 - The combustion of one mole of liquid octane,...Ch. 3 - a. Define the terms limiting reactant and excess...Ch. 3 - Define the terms theoretical yield, actual yield,...Ch. 3 -
3-75 Consider the mixture of ethanol, C2H5OH, and...Ch. 3 - Consider the mixture of propane, C3H8, and O2...Ch. 3 -
3-77 Sodium hydroxide reacts with carbon doxidc...Ch. 3 -
3.78 Aluminum hydroxide reacts with sulfuric acid...Ch. 3 - The fizz produced when an Alka-Seltzer tablet is...Ch. 3 - One of the steps in the commercial procas for...Ch. 3 - Solutions of sodium carbonate and silver nitrate...Ch. 3 - Solutions of sulfuric acid and Iead (ll) acetate...Ch. 3 - When benzene (C6H6) reacts with bromine (Br2),...Ch. 3 - When ethane (C6H6) reacts with chlorine (Cl2), the...Ch. 3 -
3.85 Hydrogen sulfide is an impurity in natural...Ch. 3 -
386 When hydrogen sulfide gas is bubbled into a...Ch. 3 -
387 Write the balanced chemical equations for
a....Ch. 3 - If 1.5 mol C2H5OH, 1.5 mol C3H8, and 1.5 mol...Ch. 3 -
3.89 The effectiveness of nitrogen fertilizers...Ch. 3 -
3.90
a. The molecular formula of acetylsalicylic...Ch. 3 - Very small semiconductor crystals, composed of...Ch. 3 - a. One molecule of the antibiotic penicillin G has...Ch. 3 - Serotonin is a compound that conducts nerve...Ch. 3 -
3.94 The koala dines exclusively on eucalyptus...Ch. 3 -
3.95 Vanillin, the dominant flavoring in vanilla,...Ch. 3 -
3.96 An organic compound was found to contain...Ch. 3 -
3.97 A compound, KBrO,, where x is unknown, is...Ch. 3 - 398 An element X forms an iodide (X13) and a...Ch. 3 - A method used by the U.S. Environmental Protection...Ch. 3 -
3.100 A chemical plant uses electrical energy to...Ch. 3 - The fat stored in a camel’s hump is a source of...Ch. 3 -
3.102 When hydrocarbons are burned in a limited...Ch. 3 -
3.103 A mixture of N2(g) and H2(g) reacts in a...Ch. 3 -
3.104 A mixture containing KClO3, K2CO3, KHCO3,...Ch. 3 - When a mixture of 10.0 g of acetylene (C2H2) and...Ch. 3 -
3.106 The semiconductor gallium arsenide, GaAs,...Ch. 3 -
3.107 Paclitaxel, C47H51NO14, is an anticancer...Ch. 3 -
3.108 Consider a sample of calcium carbonate in...Ch. 3 -
3.109
a. You are given a cube of silver metal...Ch. 3 -
3.110
a. If an automobile travels 225 mi with a...Ch. 3 - Prob. 111IECh. 3 -
3.112 A particular coal contains 2.596 sulfur by...Ch. 3 - Hydrogen cyanide, HCN, is a poisonous gas. The...Ch. 3 -
3.114 The source of oxygen that drives he...Ch. 3 -
3.115 The therrnite reaction, Fe2O3 +Al Al2O3 +...Ch. 3 -
3.116 One of the most bizarre reactions in...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 4.69 The pictures below show a molecular-scale view of a chemical reaction between H2 and CO to produce methanol, CH3OH. The box on the left represents the reactants at the instant of mixing, and the box on the right shows what is left once the reaction has gone to completion. Was there a limiting reactant in this reaction? If so, what was it? Write a balanced chemical equation for this reaction. As usual, your equation should use the smallest possible whole number coefficients for all substances.arrow_forwardPropane, C3H8, is the fuel of choice in a gas barbecue. When burning, the balanced equation is C3H8+5O23CO2+4H2O a What is the limiting reactant in cooking with a gas grill? b If the grill will not light and you know that you have an ample flow of propane to the burner, what is the limiting reactant? c When using a gas grill you can sometimes turn the gas up to the point at which the flame becomes yellow and smokey. In terms of the chemical reaction, what is happening?arrow_forward4.8 In an experiment carried out at very low pressure, 13x1015 molecules of H2 are reacted with acetylene, C2H2, to form ethane, C2H6, on the surface of a catalyst. Write a balanced chemical equation for this reaction. How many molecules of acetylene are consumed?arrow_forward
- The sugar sucrose, which is present in many fruits and vegetables, reacts in the presence of certain yeast enzymes to produce ethanol and carbon dioxide gas. Balance the following equation for this reaction of sucrose. C12H22O11(aq) + H2O(l) C2H5OH(aq) + CO2(g)arrow_forward(a) Butane gas, C4H10, can burn completely in air [use O2(g) as the other reactant] to give carbon dioxide gas and water vapor. Write a balanced equation for this combustion reaction. (b) Write a balanced chemical equation for the complete combustion of C3H7BO3, a gasoline additive. The products of combustion are CO2(g), H2O(g), and B2O3(s).arrow_forwardNitric acid is produced commercially by the Ostwald process, represented by the following equations: 4NH3(g)+5O24NO(g)+6H2O(g)2NO(g)+O2(g)2NO2(g)3NO2(g)+H2O(l)2HNO3(aq)+NO(g) What mass of NH3 must be used to produce 1.0 106 kg HNO3 by the Ostwald process? Assume 100% yield in each reaction, and assume that the NO produced in the third step is not recycled.arrow_forward
- Ammonia can be formed by a direct reaction of nitrogen and hydrogen. N2(g) + 3 H2(g) 2 NH3(g) A tiny portion of the starting mixture is represented by the diagram, where the blue circles represent N and the white circles represent H. Which of these represents the product mixture? For the reaction of the given sample, which of these statements is true? (a) N2 is the limiting reactant. (b) H2 is the limiting reactant. (c) NH, is the limiting reactant. (d) No reactant is limiting: they are present in the correct stoichiometric ratio.arrow_forwardWrite the balanced chemical equation for the complete combustion of adipic acid, an organic acid containing 49.31% C, 6.90% H, and the remainder O, by mass.arrow_forwardYou take 1.00 g of an aspirin tablet (a compound consisting solely of carbon, hydrogen, and oxygen), burn it in air, and collect 2.20 g CO2 and 0.400 g H2O. You know that the molar mass of aspirin is between 170 and 190 g/mol. Reacting 1 mole of salicylic acid with I mole of acetic anhydride (C4H6O3) gives you 1 mole of aspirin and 1 mole of acetic acid (C2H4O2). Use this information to determine the molecular formula of salicylic acid.arrow_forward
- The reaction of equal molar amounts of benzene, C6H6, and chlorine, Cl2, carried out under special conditions completely consumes the reactants and yields a gas and a clear liquid. Analysis of the liquid shows that it contains 64.03% carbon, 4.48% hydrogen, and 31.49% chlorine, and has a molar mass of 112.5 g/mol. Write the balanced equation for this reaction.arrow_forwardIn an experiment designed to produce calcium oxide by the chemical reaction 2Ca + O2 2CaO 177.2 g of CaO was obtained out of a possible 203.9 g ofCaO. a. What is the theoretical yield of CaO? b. What is the actual yield of CaO? c. What is the percent yield of CaO?arrow_forwardEthanol, C2H5OH, is a gasoline additive that can be produced by fermentation of glucose. C6H12O62C2H5OH+2CO2 (a) Calculate the mass (g) of ethanol produced by the fermentation of 1.000 lb glucose. (b) Gasohol is a mixture of 10.00 mL ethanol per 90.00 mL gasoline. Calculate the mass (in g) of glucose required to produce the ethanol in 1.00 gal gasohol. Density of ethanol = 0.785 g/mL. (c) By 2022, the U. S. Energy Independence and Security Act calls for annual production of 3.6 1010 gal of ethanol, no more than 40% of it produced by fermentation of corn. Fermentation of 1 ton (2.2 103 lb) of corn yields approximately 106 gal of ethanol. The average corn yield in the United States is about 2.1 105 lb per 1.0 105 m2. Calculate the acreage (in m2) required to raise corn solely for ethanol production in 2022 in the United States.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning
Step by Step Stoichiometry Practice Problems | How to Pass ChemistryMole Conversions Made Easy: How to Convert Between Grams and Moles; Author: Ketzbook;https://www.youtube.com/watch?v=b2raanVWU6c;License: Standard YouTube License, CC-BY