
Interpretation:
The product of the reaction for given two reactants should be drawn.
Concept introduction:
In a sigmatropic reaction “ one new sigma-bond is formed as another breaks.”
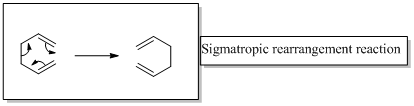
Sigma tropic rearrangement reactions are designated with digits. For example a [1, 3] sigma tropic rearrangement describe a reaction in which the residue migrates from position 1 to position 3.
Woodward –Hoffmann rules are the set of rules used to vindicate or predict certain aspects of the stereo chemical outcome and activation energy of pericyclic reactions.
Woodward – Hoffmann rules for sigma tropic rearrangement reactions are listed below
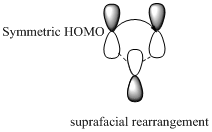
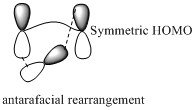
Migration of carbon and hydrogen will occur in a sigmatropic rearrangement reaction. Migration of hydrogen in suprafacial and antarafacial rearrangement can be represented as follows,


Carbon migrating with one lobe of its p orbital interacting

Carbon migrating with both lobe of its p orbital interacting

Trending nowThis is a popular solution!

Chapter 28 Solutions
EBK ORGANIC CHEMISTRY
- true or false The equilibrium constant for this reaction is 0.20. N2O4(g) ⇔ 2NO2(g) Based on the above, the equilibrium constant for the following reaction is 5. 4NO2(g) ⇔ 2N2O4(g)arrow_forwardtrue or false The equilibrium constant for this reaction is 0.20. N2O4(g) ⇔ 2NO2(g) Based on the above, the equilibrium constant for the following reaction is 0.4. 2N2O4(g) ⇔ 4NO2(g)arrow_forwardtrue or false Using the following equilibrium, if heat is added the equilibrium will shift toward the reactants. N2(g) + 3H2(g) ⇔ 2NH3(g) + heatarrow_forward
- True or False Using the following equilibrium, if heat is added the equilibrium will shift toward the products. N2O4(g) + heat ⇔ 2NO2(g)arrow_forwardtrue or false Using the following equilibrium, if solid carbon is added the equilibrium will shift toward the products. C(s) + CO2(g) ⇔ 2CO(g)arrow_forwardProvide the complete mechanism for the reaction below. You must include appropriate arrows,intermediates, and formal charges. Please also provide a reason to explain why the 1,4-adduct is preferred over the 1,3-adduct.arrow_forward
- Which of the following pairs are resonance structures of one another? I. III. || III IV + II. :0: n P !༠ IV. EN: Narrow_forwardPredict the major organic product(s) and byproducts (either organic or inorganic) for thefollowing reactions.arrow_forwardA 8.25 g sample of aluminum at 55°C released 2500 J of heat. The specific heat of aluminum is 0.900 J/g°C. The density of aluminum is 2.70 g/mL. Calculate the final temperature of the aluminum sample in °C.arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
