
(a)
Interpretation: The product of given reaction has to be drawn.
Concept introduction:
Pericyclic reactions are “ any concerted reaction in which bonds are formed or brocken in a cyclic transition state”. There is a single transition state from start to finish, in contrast to a stepwise reaction.
There are mainly three types of pericyclic reactions,
- 1) Electrocyclic reactions
- 2) Cycloaddition reactions
- 3) Sigmatropic reactions
In an electrocyclic reaction “one new sigma- bond is formed or brocken.”
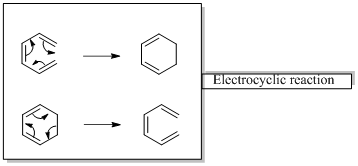
Woodward –Hoffmann rules are the set of rules used to vindicate or predict certain aspects of the stereo chemical outcome and activation energy of pericyclic reactions.
Woodward – Hoffmann rules for Electrocyclic reactions are listed below
A photochemical reaction takes place when a reactant absorbs light and a thermal reaction takes place without the absorption of light.
Woodward – Hoffmann rules for the configuration of electrocyclic reactions are,
(b)
Interpretation: The product of given reaction has to be drawn.
Concept introduction:
Pericyclic reactions are “ any concerted reaction in which bonds are formed or brocken in a cyclic transition state”. There is a single transition state from start to finish, in contrast to a stepwise reaction.
There are mainly three types of pericyclic reactions,
- 1) Electrocyclic reactions
- 2) Cycloaddition reactions
- 3) Sigmatropic reactions
In an electrocyclic reaction “one new sigma- bond is formed or brocken.”

Woodward –Hoffmann rules are the set of rules used to vindicate or predict certain aspects of the stereo chemical outcome and activation energy of pericyclic reactions.
Woodward – Hoffmann rules for Electrocyclic reactions are listed below
A photochemical reaction takes place when a reactant absorbs light and a thermal reaction takes place without the absorption of light.
Woodward – Hoffmann rules for the configuration of electrocyclic reactions are,
Want to see the full answer?
Check out a sample textbook solution
Chapter 28 Solutions
EBK ORGANIC CHEMISTRY
- Don't used hand raiting and don't used Ai solutionarrow_forward2' P17E.6 The oxidation of NO to NO 2 2 NO(g) + O2(g) → 2NO2(g), proceeds by the following mechanism: NO + NO → N₂O₂ k₁ N2O2 NO NO K = N2O2 + O2 → NO2 + NO₂ Ко Verify that application of the steady-state approximation to the intermediate N2O2 results in the rate law d[NO₂] _ 2kk₁[NO][O₂] = dt k+k₁₂[O₂]arrow_forwardPLEASE ANSWER BOTH i) and ii) !!!!arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
