
Concept explainers
(a)
Interpretation:
The name of following molecule with appropriate stereochemical designation should be determined:
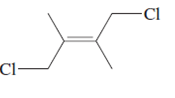
Concept introduction:
The E-configuration stands for anti-configuration, whereas, Z-configuration stands for same side configuration.
The determination of configuration is done on the basis of the
(b)
Interpretation:
The name of following molecule with appropriate stereochemical designation should be determined:
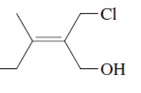
Concept introduction:
Alkenes are unsaturated hydrocarbons with double covalent bond between carbon-carbon atoms. On the basis of groups bonded with the double bonded carbon atoms, alkenes can be classified as E and Z-configuration.
The E-configuration stands for anti-configuration, whereas, Z-configuration stands for same side configuration.
The determination of configuration is done on the basis of the atomic/molecular mass of the atoms/groups attached to double bonded carbon atoms. If both higher atomic/molecular mass atom/groups are placed at the same side, then it is said to be Z-configuration and in E-configuration, these groups will be at anti-position.
(c)
Interpretation:
The name of following molecule with appropriate stereochemical designation should be determined:
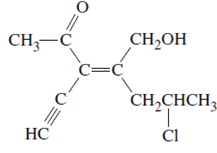
Concept introduction:
Alkenes are unsaturated hydrocarbons with double covalent bond between carbon-carbon atoms. On the basis of groups bonded with the double bonded carbon atoms, alkenes can be classified as E and Z-configuration.
The E-configuration stands for anti-configuration, whereas, Z-configuration stands for same side configuration.
The determination of configuration is done on the basis of the atomic/molecular mass of the atoms/groups attached to double bonded carbon atoms. If both higher atomic/molecular mass atom/groups are placed at the same side, then it is said to be Z-configuration and in E-configuration, these groups will be at anti-position.
(d)
Interpretation:
The name of following molecule with appropriate stereochemical designation should be determined:
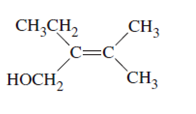
Concept introduction:
Alkenes are unsaturated hydrocarbons with double covalent bond between carbon-carbon atoms. On the basis of groups bonded with the double bonded carbon atoms, alkenes can be classified as E and Z-configuration.
The E-configuration stands for anti-configuration, whereas, Z-configuration stands for same side configuration.
The determination of configuration is done on the basis of the atomic/molecular mass of the atoms/groups attached to double bonded carbon atoms. If both higher atomic/molecular mass atom/groups are placed at the same side, then it is said to be Z-configuration and in E-configuration, these groups will be at anti-position.
Want to see the full answer?
Check out a sample textbook solution
Chapter 26 Solutions
EBK GENERAL CHEMISTRY
- Don't used hand raiting and don't used Ai solutionarrow_forward2' P17E.6 The oxidation of NO to NO 2 2 NO(g) + O2(g) → 2NO2(g), proceeds by the following mechanism: NO + NO → N₂O₂ k₁ N2O2 NO NO K = N2O2 + O2 → NO2 + NO₂ Ко Verify that application of the steady-state approximation to the intermediate N2O2 results in the rate law d[NO₂] _ 2kk₁[NO][O₂] = dt k+k₁₂[O₂]arrow_forwardPLEASE ANSWER BOTH i) and ii) !!!!arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
