
Indicate whether each of the following aspects of triacylglycerol digestion is associated with the (1) mouth, (2) stomach, (3) small intestine, (4) intestinal cells, or (5) bloodstream.
- a. Chyme is produced.
- b. Gastric lipases are active.
- c. Initial production of monoacylglycerols occurs.
- d. Fatty acid micelles are formed.
(a)
Interpretation: To identify whether the chyme is produced in the (1) mouth (2) stomach (3) small intestine, (4) intestinal cells, or (5) bloodstream.
Concept introduction: In the stomach, the muscle walls of the stomach churn the food and other complex molecules into smaller particles. The churning action breaks up the lipid molecules into small droplets/globules which remains suspended on the other smaller components of the ingested food. This results in the formation of a semi-liquid material comprising of small lipid droplets, partly digested food and gastric secretions called chyme. The chyme, thereafter, resumes its journey to the small intestine.
Answer to Problem 25.1EP
Chyme production occurs in the stomach.
Explanation of Solution
Chyme is a semi-liquid material composed of small lipid droplets, partly digested food and gastric secretions. Since the initial breakdown of lipid molecules and activity of the gastric lipases begins in the stomach, hence the formation of chyme occurs in the stomach.
(b)
Interpretation: To identify whether the gastric lipase is active in (1) mouth (2) stomach (3) small intestine, (4) intestinal cells, or (5) bloodstream.
Concept introduction: Gastric lipase is an acidic lipase secreted by the gastric chief cells in the fundic mucosa in the stomach. The function of the enzyme is to break down lipid molecules. Gastric lipases are enzymes which break down lipids.
Answer to Problem 25.1EP
Gastric lipases are active in the stomach.
Explanation of Solution
Gastric lipases are present in the stomach and help in the initial hydrolysis of the triacylglycerol molecules. The activity of the gastric lipases in the stomach leads to the initial hydrolysis of lipid molecules (triacylglycerol). Around 10% of the triacylglycerols are hydrolyzed in the stomach by the action of gastric lipases. Hence, gastric lipases are active in the stomach.
The chyme from the stomach enters the small intestine. The bile juice is released from the gall bladder into the small intestine. The digestion of lipid continues and triacylglycerol molecules are broken down into monoacylglycerol and free fatty acids.
(c)
Interpretation: To identify whether the initial production of monoacylglycerol occurs in (1) mouth (2) stomach (3) small intestine, (4) intestinal cells, or (5) bloodstream.
Concept introduction: The initial lipid digestion begins in the stomach in the presence of gastric lipases. Gastric lipases are enzymes which break down lipids. The activity of the gastric lipases in the stomach leads to the initial hydrolysis of lipid molecules (triacylglycerol). The chyme from the stomach enters the small intestine. The bile juice is released from the gall bladder into the small intestine. Here the digestion of lipid continues and triacylglycerol molecules are broken down into monoacylglycerol and free fatty acids.
Answer to Problem 25.1EP
The initial production of monoacylglycerol occurs in the stomach.
Explanation of Solution
In the stomach, around 10% of the triacylglycerol is hydrolyzed into monoacylglycerol and free fatty acids under the activity of gastric lipases. The remaining hydrolysis occurs in the small intestine. Hence, the initial production of monoacylglycerol occurs in the stomach.
(d)
Interpretation: To identify whether the fatty acid micelles are formed in the (1) mouth (2) stomach (3) small intestine, (4) intestinal cells, or (5) bloodstream.
Concept introduction: Fatty acid is a carboxylic acid that contains a long hydrophobic hydrocarbon chain and a polar carboxyl group. The fatty acids are aligned such that the hydrophobic hydrocarbon chain is directed inwards away from the polar environment and the polar carboxyl group head is directed outwards. The aggregate thus formed is called micelle.
A general representation of fatty acid is,
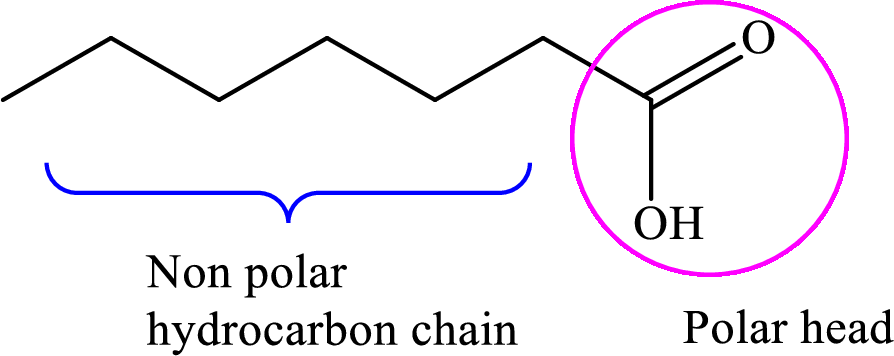
A diagrammatic representation of micelle is as follows:
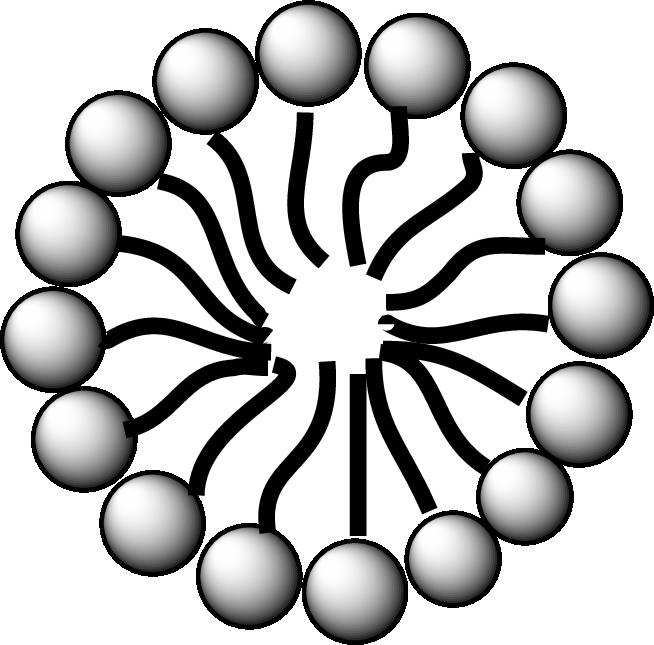
Answer to Problem 25.1EP
Fatty acid micelles are formed in the small intestine.
Explanation of Solution
A fatty acid micelle is a spherical droplet which is primarily composed of free fatty acids, monoacylglycerols, and bile.
The chyme from the stomach enters the small intestine. The bile juice is released from the gall bladder into the small intestine. The digestion of lipid continues and triacylglycerol molecules are broken down into monoacylglycerol and free fatty acids.
In the small intestine, the monoacylglycerols and free fatty acids along with bile and pancreatic lipases, form micelles. Micelles are later absorbed by the cell membranes of the intestinal cells.
Want to see more full solutions like this?
Chapter 25 Solutions
EBK GENERAL, ORGANIC, AND BIOLOGICAL CH
- HELP! URGENT! PLEASE RESOND ASAP!arrow_forwardQuestion 4 Determine the rate order and rate constant for sucrose hydrolysis. Time (hours) [C6H12O6] 0 0.501 0.500 0.451 1.00 0.404 1.50 0.363 3.00 0.267 First-order, k = 0.210 hour 1 First-order, k = 0.0912 hour 1 O Second-order, k = 0.590 M1 hour 1 O Zero-order, k = 0.0770 M/hour O Zero-order, k = 0.4896 M/hour O Second-order, k = 1.93 M-1-hour 1 10 ptsarrow_forwardDetermine the rate order and rate constant for sucrose hydrolysis. Time (hours) [C6H12O6] 0 0.501 0.500 0.451 1.00 0.404 1.50 0.363 3.00 0.267arrow_forward
- Draw the products of the reaction shown below. Use wedge and dash bonds to indicate stereochemistry. Ignore inorganic byproducts. OSO4 (cat) (CH3)3COOH Select to Draw ઘarrow_forwardCalculate the reaction rate for selenious acid, H2SeO3, if 0.1150 M I-1 decreases to 0.0770 M in 12.0 minutes. H2SeO3(aq) + 6I-1(aq) + 4H+1(aq) ⟶ Se(s) + 2I3-1(aq) + 3H2O(l)arrow_forwardProblem 5-31 Which of the following objects are chiral? (a) A basketball (d) A golf club (b) A fork (c) A wine glass (e) A spiral staircase (f) A snowflake Problem 5-32 Which of the following compounds are chiral? Draw them, and label the chirality centers. (a) 2,4-Dimethylheptane (b) 5-Ethyl-3,3-dimethylheptane (c) cis-1,4-Dichlorocyclohexane Problem 5-33 Draw chiral molecules that meet the following descriptions: (a) A chloroalkane, C5H11Cl (c) An alkene, C6H12 (b) An alcohol, C6H140 (d) An alkane, C8H18 Problem 5-36 Erythronolide B is the biological precursor of erythromycin, a broad-spectrum antibiotic. How H3C CH3 many chirality centers does erythronolide B have? OH Identify them. H3C -CH3 OH Erythronolide B H3C. H3C. OH OH CH3arrow_forward
- Problem 5-48 Assign R or S configurations to the chirality centers in ascorbic acid (vitamin C). OH H OH HO CH2OH Ascorbic acid O H Problem 5-49 Assign R or S stereochemistry to the chirality centers in the following Newman projections: H Cl H CH3 H3C. OH H3C (a) H H H3C (b) CH3 H Problem 5-52 Draw the meso form of each of the following molecules, and indicate the plane of symmetry in each: OH OH (a) CH3CHCH2CH2CHCH3 CH3 H3C. -OH (c) H3C CH3 (b) Problem 5-66 Assign R or S configurations to the chiral centers in cephalexin, trade-named Keflex, the most widely prescribed antibiotic in the United States. H2N H IHH S Cephalexin N. CH3 CO₂Harrow_forwardSteps and explanationn please.arrow_forwardSteps and explanationn please.arrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Chemistry In FocusChemistryISBN:9781305084476Author:Tro, Nivaldo J., Neu, Don.Publisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry In FocusChemistryISBN:9781305084476Author:Tro, Nivaldo J., Neu, Don.Publisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co





