
PHYSICS FOR SCIEN & ENGNR W/MOD MAST
4th Edition
ISBN: 9780134112039
Author: GIANCOLI
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 24, Problem 69P
(III) The capacitor shown in Fig. 24–34 is connected to a 90.0-V battery. Calculate (and sketch) the electric field everywhere between the capacitor plates. Find both the free charge on the capacitor plate and the induced charge on the faces of the glass dielectric plate.
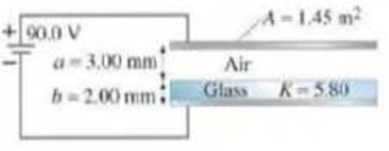
FIGURE 24–34 Problem 69.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
A mass is connect to a vertical revolving axle by two strings of length L, each making an angle of 45 degrees with the axle, as shown. Both the axle and mass are revolving with angular velocity w, Gravity is directed downward. The tension in the upper string is T_upper and the tension in the lower string is T_lower.Draw a clear free body diagram for mass m. Please include real forces only.Find the tensions in the upper and lower strings, T_upper and T_lower
2. A stone is dropped into a pool of water causing ripple to spread out. After 10 s
the circumference of the ripple is 20 m. Calculate the velocity of the wave.
10. Imagine you have a system in which you have 54 grams of ice. You can melt this
ice and then vaporize it all at 0 C. The melting and vaporization are done reversibly
into a balloon held at a pressure of 0.250 bar. Here are some facts about water you
may wish to know. The density of liquid water at 0 C is 1 g/cm³. The density of ice at 0
C is 0.917 g/cm³. The enthalpy of vaporization of liquid water is 2.496 kJ/gram and the
enthalpy of fusion of solid water is 333.55 J/gram.
A. How much energy does the ice absorb as heat when it melts?
B. How much work is involved in melting the ice?
C. What is the total change in energy for melting the ice?
D. What is the enthalpy change for melting the ice?
E. What is the entropy change for melting the ice?
F. What is the change in Helmholtz energy for melting the ice?
G. What is the change in Gibbs energy for melting the ice?
Chapter 24 Solutions
PHYSICS FOR SCIEN & ENGNR W/MOD MAST
Ch. 24.1 - Graphs for charge versus voltage are shown in Fig....Ch. 24.2 - Two circular plates of radius 5.0 cm are separated...Ch. 24.2 - What is the capacitance per unit length of a...Ch. 24.3 - Consider two identical capacitors C1 = C2 = 10 F....Ch. 24.5 - Return to the Chapter-Opening Question, page 628,...Ch. 24 - Suppose two nearby conductors carry the same...Ch. 24 - Suppose the separation of plates d in a...Ch. 24 - Suppose one of the plates of a parallel-plate...Ch. 24 - When a battery is connected to a capacitor, why do...Ch. 24 - Describe a sample method of measuring 0 using a...
Ch. 24 - Suppose three identical capacitors are connected...Ch. 24 - A large copper sheet of thickness is placed...Ch. 24 - The parallel plates of an isolated capacitor carry...Ch. 24 - How does the energy in a capacitor change if (a)...Ch. 24 - If the voltage across a capacitor is doubled, the...Ch. 24 - An isolated charged capacitor has horizontal...Ch. 24 - Suppose a battery remains connected to the...Ch. 24 - How does the energy stored in a capacitor change...Ch. 24 - For dielectrics consisting of polar molecules, how...Ch. 24 - A dielectric is pulled out from between the plates...Ch. 24 - We have seen that the capacitance C depends on the...Ch. 24 - What value might we assign to the dielectric...Ch. 24 - (I) The two plates of a capacitor hold +2800 C and...Ch. 24 - (I) How much charge flows from a 12.0-V battery...Ch. 24 - (I) The potential difference between two short...Ch. 24 - (I) The charge on a capacitor increases by 26 C...Ch. 24 - (II) A 7.7-F capacitor is charged by a 125-V...Ch. 24 - (II) An isolated capacitor C1 carries a charge Q0....Ch. 24 - (II) It takes 15 J of energy to move a 0.20-mC...Ch. 24 - (II) A 2.70-F capacitor is charged to 475 V and a...Ch. 24 - (II) Compact ultracapacitors with capacitance...Ch. 24 - (II) In a dynamic random access memory (DRAM)...Ch. 24 - (I) To make a 0.40-F capacitor, what area must the...Ch. 24 - (I) What is the capacitance per unit length (F/m)...Ch. 24 - (I) Determine the capacitance of the Earth,...Ch. 24 - (II) Use Gausss law to show that E=0 inside the...Ch. 24 - (II) Dry air will break down if the electric field...Ch. 24 - (II) An electric field of 4.80 105V/m is desired...Ch. 24 - (II) How strong is the electric field between the...Ch. 24 - (II) A large metal sheet of thickness is placed...Ch. 24 - (III) Small distances are commonly measured...Ch. 24 - (III) In an electrostatic air cleaner...Ch. 24 - (I) The capacitance of a portion of a circuit is...Ch. 24 - (I) (a) Six 3.8-F capacitors are connected in...Ch. 24 - (II) Given three capacitors, C1 = 2.0 F, C2 = 1.5...Ch. 24 - (II) Suppose three parallel-plate capacitors,...Ch. 24 - (II) An electric circuit was accidentally...Ch. 24 - (II) Three conducting plates, each of area A, are...Ch. 24 - (II) Consider three capacitors, of capacitance...Ch. 24 - (II) A 0.50-F and a 0.80-F capacitor are connected...Ch. 24 - (II) In Fig. 2423, suppose C1 = C2 = C3 = C4 = C....Ch. 24 - (II) Suppose in Fig. 2423 that C1 = C2 = C3 = 16.0...Ch. 24 - (II) The switch S in Mg. 2424 is connected...Ch. 24 - (II) (a) Determine the equivalent capacitance...Ch. 24 - FIGURE 2425 Problems 32 and 33. (II) Suppose in...Ch. 24 - (II) Two capacitors connected in parallel produce...Ch. 24 - (II) In the capacitance bridge shown m Fig. 2426,...Ch. 24 - (II) Two capacitors, C1 = 3200 pF and C2 = 1800...Ch. 24 - (II) (a) Determine the equivalent capacitance of...Ch. 24 - (II) In Fig. 2427, let C1 = 2.00 F, C2 = 3.00 F,...Ch. 24 - (III) Suppose one plate of a parallel-plate...Ch. 24 - (III) A voltage V is applied to the capacitor...Ch. 24 - (I) 2200 V is applied to a 2800-pF capacitor. How...Ch. 24 - (I) There is an electric field near the Earths...Ch. 24 - (I) How much energy is stored by the electric...Ch. 24 - (II) A parallel-plate capacitor has fixed charges...Ch. 24 - (II) In Fig. 2427, Let V = 10.0 V and C1 = C2 = C3...Ch. 24 - (II) How much energy must a 28-V battery expend to...Ch. 24 - (II) (a) Suppose the outer radius Ra of a...Ch. 24 - (II) A 2.2-F capacitor is charged by a 12.0-V...Ch. 24 - (II) How much work would be required to remove a...Ch. 24 - (II) (a) Show that each plate of a parallel-plate...Ch. 24 - (II) Show that the electrostatic energy stored in...Ch. 24 - (II) When two capacitors are connected in parallel...Ch. 24 - (II) For commonly used CMOS (complementary metal...Ch. 24 - (I) What is the capacitance of two square parallel...Ch. 24 - (II) Suppose the capacitor in Example 2411 remains...Ch. 24 - (II) How much energy would be stored in the...Ch. 24 - (II) In the DRAM computer chip of Problem 10, the...Ch. 24 - (II) A 3500-pF air-gap capacitor is connected to a...Ch. 24 - (II) Two different dielectrics each fill half the...Ch. 24 - (II) Two different dielectrics fill the space...Ch. 24 - (II) Repeat Problem 60 (Fig. 2431) but assume the...Ch. 24 - (II) Two identical capacitors are connected in...Ch. 24 - (III) A slab of width d and dielectric constant K...Ch. 24 - (III) The quantity of liquid (such as cryogenic...Ch. 24 - (II) Show that the capacitor in Example 2412 with...Ch. 24 - (II) Repeat Example 24-12 assuming the battery...Ch. 24 - (II) Using Example 2412 as a model, derive a...Ch. 24 - (II) In Example 2412 what percent of the stored...Ch. 24 - (III) The capacitor shown in Fig. 2434 is...Ch. 24 - (a) A general rule for estimating the capacitance...Ch. 24 - A cardiac defibrillator is used to shock a heart...Ch. 24 - A homemade capacitor is assembled by placing two...Ch. 24 - An uncharged capacitor is connected to a 34.0-V...Ch. 24 - It takes 18.5 J of energy to move a 13.0-mC charge...Ch. 24 - A huge 3.0-F capacitor has enough stored energy to...Ch. 24 - A coaxial cable, Fig. 2435, consists of an inner...Ch. 24 - The electric field between the plates of a...Ch. 24 - Capacitors can be used as electric charge...Ch. 24 - A parallel-plate capacitor is isolated with a...Ch. 24 - In lightning storms, the potential difference...Ch. 24 - A multilayer film capacitor has a maximum voltage...Ch. 24 - A 3.5 F capacitor is charged by a 12.4-V battery...Ch. 24 - The power supply for a pulsed nitrogen laser has a...Ch. 24 - A parallel-plate capacitor has square plates 12 cm...Ch. 24 - The variable capacitance of an old radio tuner...Ch. 24 - A high-voltage supply can be constructed from a...Ch. 24 - A 175-pF capacitor is connected in series with an...Ch. 24 - A parallel-plate capacitor with plate area 2.0 cm2...Ch. 24 - In the circuit shown in Fig. 2437. C1 = 1.0 F, C2...Ch. 24 - The long cylindrical capacitor shown in Fig. 2438...Ch. 24 - A parallel-plate capacitor has plate area A, plate...Ch. 24 - Consider the use of capacitors as memory cells. A...Ch. 24 - To get an idea how big a farad is, suppose you...Ch. 24 - A student wearing shoes with thin insulating soles...Ch. 24 - A parallel-plate capacitor with plate area A = 2.0...Ch. 24 - Let us try to estimate the maximum static...Ch. 24 - Paper has a dielectric constant K = 3.7 and a...Ch. 24 - (II) Six physics students were each given an air...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Researchers cross a corn plant that is pure - breeding forthe dominant traits colored aleurone (C1), full kerne...
Genetic Analysis: An Integrated Approach (3rd Edition)
5. When the phenotype of heterozygotes is intermediate between the phenotypes of the two homozygotes, this patt...
Biology: Life on Earth (11th Edition)
Which one of the following is not a fuel produced by microorganisms? a. algal oil b. ethanol c. hydrogen d. met...
Microbiology: An Introduction
11. A 1.0-cm-thick layer of water stands on a horizontal slab of glass. A light ray in the air is incident on t...
College Physics: A Strategic Approach (3rd Edition)
Why is petroleum jelly used in the hanging-drop procedure?
Laboratory Experiments in Microbiology (12th Edition) (What's New in Microbiology)
The pHactivity profile for glucose-6-phosphate isomerase indicates the participation of a group with a pKa = 6....
Organic Chemistry (8th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- In the figure Q = 5.7 nC and all other quantities are accurate to 2 significant figures. What is the magnitude of the force on the charge Q? (k = 1/4πε 0 = 8.99 × 109 N · m2/C2)arrow_forwardNow add a fourth charged particle, particle 3, with positive charge q3, fixed in the yz-plane at (0,d2,d2). What is the net force F→ on particle 0 due solely to this charge? Express your answer (a vector) using k, q0, q3, d2, i^, j^, and k^. Include only the force caused by particle 3.arrow_forwardFor a tornadoes and hurricanes, which of the following is most critical? an alert a watch a warning a predictionarrow_forward
- When a warm front advances up and over a cold front, what is it called? front inversion stationary front cold front occlusion warm front occlusionarrow_forward1) Consider two positively charged particles, one of charge q0 (particle 0) fixed at the origin, and another of charge q1 (particle 1) fixed on the y-axis at (0,d1,0). What is the net force F→ on particle 0 due to particle 1? Express your answer (a vector) using any or all of k, q0, q1, d1, i^, j^, and k^. 2) Now add a third, negatively charged, particle, whose charge is −q2− (particle 2). Particle 2 fixed on the y-axis at position (0,d2,0). What is the new net force on particle 0, from particle 1 and particle 2? Express your answer (a vector) using any or all of k, q0, q1, q2, d1, d2, i^, j^, and k^. 3) Particle 0 experiences a repulsion from particle 1 and an attraction toward particle 2. For certain values of d1 and d2, the repulsion and attraction should balance each other, resulting in no net force. For what ratio d1/d2 is there no net force on particle 0? Express your answer in terms of any or all of the following variables: k, q0, q1, q2.arrow_forwardA 85 turn, 10.0 cm diameter coil rotates at an angular velocity of 8.00 rad/s in a 1.35 T field, starting with the normal of the plane of the coil perpendicular to the field. Assume that the positive max emf is reached first. (a) What (in V) is the peak emf? 7.17 V (b) At what time (in s) is the peak emf first reached? 0.196 S (c) At what time (in s) is the emf first at its most negative? 0.589 x s (d) What is the period (in s) of the AC voltage output? 0.785 Sarrow_forward
- A bobsled starts at the top of a track as human runners sprint from rest and then jump into the sled. Assume they reach 40 km/h from rest after covering a distance of 50 m over flat ice. a. How much work do they do on themselves and the sled which they are pushing given the fact that there are two men of combined mass 185 kg and the sled with a mass of 200 kg? (If you haven't seen bobsledding, watch youtube to understand better what's going on.) b. After this start, the team races down the track and descends vertically by 200 m. At the finish line the sled crosses with a speed of 55 m/s. How much energy was lost to drag and friction along the way down after the men were in the sled?arrow_forwardFor what type of force is it not possible to define a potential energy expression?arrow_forward10. Imagine you have a system in which you have 54 grams of ice. You can melt this ice and then vaporize it all at 0 C. The melting and vaporization are done reversibly into a balloon held at a pressure of 0.250 bar. Here are some facts about water you may wish to know. The density of liquid water at 0 C is 1 g/cm³. The density of ice at 0 C is 0.917 g/cm³. The enthalpy of vaporization of liquid water is 2.496 kJ/gram and the enthalpy of fusion of solid water is 333.55 J/gram.arrow_forward
- Consider 1 mole of supercooled water at -10°C. Calculate the entropy change of the water when the supercooled water freezes at -10°C and 1 atm. Useful data: Cp (ice) = 38 J mol-1 K-1 Cp (water) 75J mol −1 K -1 Afus H (0°C) 6026 J mol −1 Assume Cp (ice) and Cp (water) to be independent of temperature.arrow_forwardThe molar enthalpy of vaporization of benzene at its normal boiling point (80.09°C) is 30.72 kJ/mol. Assuming that AvapH and AvapS stay constant at their values at 80.09°C, calculate the value of AvapG at 75.0°C, 80.09°C, and 85.0°C. Hint: Remember that the liquid and vapor phases will be in equilibrium at the normal boiling point.arrow_forward3. The entropy of an ideal gas is S = Nkg In V. Entropy is a state function rather than a path function, and in this problem, you will show an example of the entropy change for an ideal gas being the same when you go between the same two states by two different pathways. A. Express ASV = S2 (V2) - S₁(V1), the change in entropy upon changing the volume from V₁to V2, at fixed particle number N and energy, U. B. Express ASN = S₂(N₂) - S₁ (N₁), the change in entropy upon changing the particle number from N₁ to N2, at fixed volume V and energy U. C. Write an expression for the entropy change, AS, for a two-step process (V₁, N₁) → (V2, N₁) → (V2, N₂) in which the volume changes first at fixed particle number, then the particle number changes at fixed volume. Again, assume energy is constant.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning Glencoe Physics: Principles and Problems, Student...PhysicsISBN:9780078807213Author:Paul W. ZitzewitzPublisher:Glencoe/McGraw-Hill
Glencoe Physics: Principles and Problems, Student...PhysicsISBN:9780078807213Author:Paul W. ZitzewitzPublisher:Glencoe/McGraw-Hill College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College
College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College Classical Dynamics of Particles and SystemsPhysicsISBN:9780534408961Author:Stephen T. Thornton, Jerry B. MarionPublisher:Cengage Learning
Classical Dynamics of Particles and SystemsPhysicsISBN:9780534408961Author:Stephen T. Thornton, Jerry B. MarionPublisher:Cengage Learning


Physics for Scientists and Engineers: Foundations...
Physics
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Cengage Learning

College Physics
Physics
ISBN:9781285737027
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

Glencoe Physics: Principles and Problems, Student...
Physics
ISBN:9780078807213
Author:Paul W. Zitzewitz
Publisher:Glencoe/McGraw-Hill

College Physics
Physics
ISBN:9781938168000
Author:Paul Peter Urone, Roger Hinrichs
Publisher:OpenStax College

Classical Dynamics of Particles and Systems
Physics
ISBN:9780534408961
Author:Stephen T. Thornton, Jerry B. Marion
Publisher:Cengage Learning
Physics Capacitor & Capacitance part 7 (Parallel Plate capacitor) CBSE class 12; Author: LearnoHub - Class 11, 12;https://www.youtube.com/watch?v=JoW6UstbZ7Y;License: Standard YouTube License, CC-BY