
General Chemistry: Principles and Modern Applications (11th Edition)
11th Edition
ISBN: 9780132931281
Author: Ralph H. Petrucci, F. Geoffrey Herring, Jeffry D. Madura, Carey Bissonnette
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 23, Problem 86SAE
Provide the missing name or formula for the following. 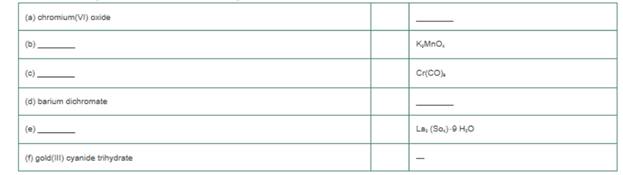
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Don't used hand raiting and don't used Ai solution
Don't used Ai solution and don't used hand raiting
OA. For the structure shown, rank the bond lengths (labeled a, b and c) from shortest to longest. Place your answer in
the box. Only the answer in the box will be graded. (2 points)
H
-CH3
THe
b
Н
Chapter 23 Solutions
General Chemistry: Principles and Modern Applications (11th Edition)
Ch. 23 - By means of orbital diagrams, write electron...Ch. 23 - Arrange the following species according to the...Ch. 23 - Describe how the transition elements compare with...Ch. 23 - With only minor irregularities, the melting points...Ch. 23 - Why do the atomic radii vary so much for two...Ch. 23 - Prob. 6ECh. 23 - Which of me first transition series elements...Ch. 23 - Why is the number of common oxidation states for...Ch. 23 - As a group, the lanthanides are more reactive...Ch. 23 - The maximum difference in standard reduction...
Ch. 23 - Complete and balance the following equations. If...Ch. 23 - By means of e chemical equation, give an example...Ch. 23 - Prob. 13ECh. 23 - Prob. 14ECh. 23 - Prob. 15ECh. 23 - Prob. 16ECh. 23 - Prob. 17ECh. 23 - According to Figure 23-8, G decreases with...Ch. 23 - Prob. 19ECh. 23 - Prob. 20ECh. 23 - Write plausible half-equations to represent each...Ch. 23 - Prob. 22ECh. 23 - Use electrode potential data from this chapter or...Ch. 23 - You are given these three reducing agents: Zn(s);...Ch. 23 - Prob. 25ECh. 23 - Prob. 26ECh. 23 - Prob. 27ECh. 23 - Use data from the text to construct a standard...Ch. 23 - When a soluble lead compound is added to a...Ch. 23 - Prob. 30ECh. 23 - Prob. 31ECh. 23 - If CO2(g) under pressure is passed into...Ch. 23 - Use equation (23.19) to determine [Cr2O72] in a...Ch. 23 - If a solution is prepared by dissolving 1.505 g...Ch. 23 - Prob. 35ECh. 23 - Prob. 36ECh. 23 - Prob. 37ECh. 23 - Prob. 38ECh. 23 - Will reaction (23.25) still be spontaneous in the...Ch. 23 - Prob. 40ECh. 23 - Prob. 41ECh. 23 - Prob. 42ECh. 23 - Prob. 43ECh. 23 - Prob. 44ECh. 23 - Prob. 45ECh. 23 - Prob. 46ECh. 23 - Prob. 47ECh. 23 - At 400C , 2Hg(I)+O2(g)2HgO(s) for the reaction...Ch. 23 - Prob. 49ECh. 23 - Prob. 50ECh. 23 - Prob. 51ECh. 23 - Prob. 52ECh. 23 - Prob. 53ECh. 23 - Prob. 54ECh. 23 - The text notes that in small quantities, zinc is...Ch. 23 - Prob. 56ECh. 23 - What formulas would you expect for the metal...Ch. 23 - For the straight-line graphs in Figure 23-8...Ch. 23 - Prob. 59ECh. 23 - Prob. 60ECh. 23 - Prob. 61ECh. 23 - Prob. 62ECh. 23 - Prob. 63ECh. 23 - Prob. 64ECh. 23 - Prob. 65ECh. 23 - Prob. 66ECh. 23 - Prob. 67ECh. 23 - Prob. 68ECh. 23 - Prob. 69ECh. 23 - Prob. 70ECh. 23 - Prob. 71ECh. 23 - For a coordination number of four, the radius of...Ch. 23 - Prob. 73ECh. 23 - Prob. 74FPCh. 23 - Several transition metal ions are found in cation...Ch. 23 - Prob. 76SAECh. 23 - Briefly describe each of the following ideas. phe...Ch. 23 - Prob. 78SAECh. 23 - Prob. 79SAECh. 23 - Prob. 80SAECh. 23 - Prob. 81SAECh. 23 - Prob. 82SAECh. 23 - Prob. 83SAECh. 23 - Prob. 84SAECh. 23 - Prob. 85SAECh. 23 - Provide the missing name or formula for the...Ch. 23 - Prob. 87SAECh. 23 - Prob. 88SAECh. 23 - Prob. 89SAECh. 23 - Prob. 90SAE
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Don't used hand raitingarrow_forwardQuizzes - Gen Organic & Biological Che... ☆ myd21.lcc.edu + O G screenshot on mac - Google Search savings hulu youtube google disney+ HBO zlib Homework Hel...s | bartleby cell bio book Yuzu Reader: Chemistry G periodic table - Google Search b Home | bartleby 0:33:26 remaining CHEM 120 Chapter 5_Quiz 3 Page 1: 1 > 2 > 3 > 6 ¦ 5 > 4 > 7 ¦ 1 1 10 8 ¦ 9 a ¦ -- Quiz Information silicon-27 A doctor gives a patient 0.01 mC i of beta radiation. How many beta particles would the patient receive in I minute? (1 Ci = 3.7 x 10 10 d/s) Question 5 (1 point) Saved Listen 2.22 x 107 222 x 108 3.7 x 108 2.22 x 108 none of the above Question 6 (1 point) Listen The recommended dosage of 1-131 for a test is 4.2 μCi per kg of body mass. How many millicuries should be given to a 55 kg patient? (1 mCi = 1000 μСi)? 230 mCiarrow_forwardDon't used hand raiting and don't used Ai solutionarrow_forward
- Don't used hand raiting and don't used Ai solutionarrow_forwardQ3: Arrange each group of compounds from fastest SN2 reaction rate to slowest SN2 reaction rate. CI Cl H3C-Cl CI a) A B C D Br Br b) A B C Br H3C-Br Darrow_forwardQ4: Rank the relative nucleophilicity of halide ions in water solution and DMF solution, respectively. F CI Br | Q5: Determine which of the substrates will and will not react with NaSCH3 in an SN2 reaction to have a reasonable yield of product. NH2 Br Br Br .OH Brarrow_forward
- Classify each molecule as optically active or inactive. Determine the configuration at each H соон Chirality center OH 애 He OH H3C Ноос H H COOH A K B.arrow_forwardQ1: Rank the relative nucleophilicity of the following species in ethanol. CH3O¯, CH3OH, CH3COO, CH3COOH, CH3S Q2: Group these solvents into either protic solvents or aprotic solvents. Acetonitrile (CH3CN), H₂O, Acetic acid (CH3COOH), Acetone (CH3COCH3), CH3CH2OH, DMSO (CH3SOCH3), DMF (HCON(CH3)2), CH3OHarrow_forwardDon't used hand raiting and don't used Ai solutionarrow_forward
- 10. The main product of the following reaction is [1.1:4',1"-terphenyl]-2'-yl(1h-pyrazol-4- yl)methanone Ph N-H Pharrow_forwardDraw the Fischer projection for a D-aldo-pentose. (aldehyde pentose). How many total stereoisomers are there? Name the sugar you drew. Draw the Fischer projection for a L-keto-hexose. (ketone pentose). How many total stereoisomers are there? Draw the enantiomer.arrow_forwardDraw a structure using wedges and dashes for the following compound: H- Et OH HO- H H- Me OHarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning
Stoichiometry - Chemistry for Massive Creatures: Crash Course Chemistry #6; Author: Crash Course;https://www.youtube.com/watch?v=UL1jmJaUkaQ;License: Standard YouTube License, CC-BY
Bonding (Ionic, Covalent & Metallic) - GCSE Chemistry; Author: Science Shorts;https://www.youtube.com/watch?v=p9MA6Od-zBA;License: Standard YouTube License, CC-BY
General Chemistry 1A. Lecture 12. Two Theories of Bonding.; Author: UCI Open;https://www.youtube.com/watch?v=dLTlL9Z1bh0;License: CC-BY