
Concept explainers
Synthetic Applications of Enamines
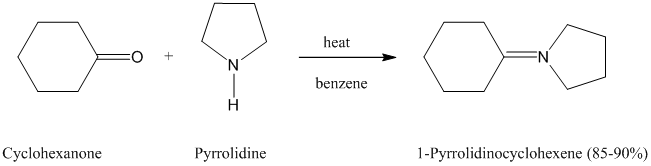
The formation of enamines by the reaction of
described in Section

When preparing enamines, the reaction is normally carried out by heating in benzene as the solvent. No catalyst is necessary, but
Enamines resemble enols in that electron-pair donation makes their double bond electron-rich
and nucleophilic.
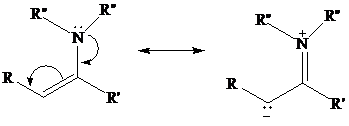
Because nitrogen is a better electron-pair donor than oxygen, an enamine is more nucleophilic thanan enol. Enamines, being neutral molecules, are, however, less nucleophilic than enolates, which areanions.
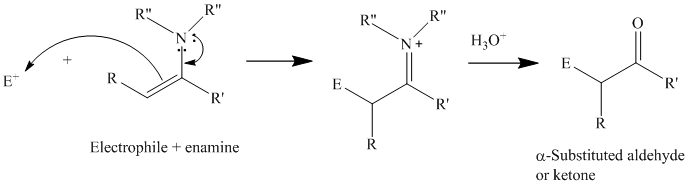
Reactions of enamines with electrophiles
Pyrrolidine is the secondary amine used most often for making enamines from aldehydes and
ketones.
For synthetic purposes, the electrophilic reagents that give the best yields of
Acyl chlorides and acid anhydrides.
Michael acceptors:
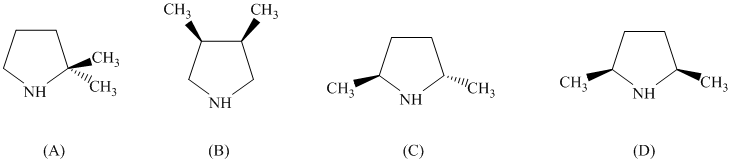
pyrrolidine derivative. Of the following, which one is the best pyrrolidine derivative to use
in this enantioselective synthesis?
Want to see the full answer?
Check out a sample textbook solution
Chapter 22 Solutions
ORGANIC CHEMISTRY-W/STUD.SOLN.MAN.
- From your calculations, which reaction experiment had closest to stoichiometric quantities? How many moles of NaHCO3 and HC2H3O2 were present in this reaction?arrow_forward18. Arrange the following carbocations in order of decreasing stability. 1 2 A 3124 B 4213 C 2431 D 1234 E 2134 SPL 3 4arrow_forwardAcetic acid is added to DI water at an initial concentration of 10 -6 M (Ka=1.8x10-5) A. Using the "ICE" Method, what would the pH be at equilibrium? State assumptions and show your work. B. Using the simultaneous equations method, what would the pH be at equilibrium? Show your workarrow_forward
- 1. Show that the change in entropy for a fixed amount of ideal gas held at a constant temperature undergoing a volume change is given by the simple equation AS = NkB In Hint: Start with the equation M dS = du + (Œ) dv - Ž (#) an, dU du+av-dN; j=1 Why doesn't the equation for the entropy of an ideal gas depend on the strength of the intermolecular forces for the gas?arrow_forward2. Make an ice cube at 1 bar pressure by freezing an amount of liquid water that is 2 cm x 2 cm x 2 cm in volume. The density of liquid water at 0 °C is 1.000 g cm³ and the density of ice at 0 °C is 0.915 g cm³. Note that this difference in density is the reason your water pipes burst if they freeze and why you shouldn't forget to take your bottle of pop out of the freezer if you put it in there to try and cool it down faster. A. What is the work of expansion upon freezing? B. Is work done on the system or by the system?arrow_forwardI have a excitation/emission spectra of a quinine standard solution here, and I'm having trouble interpreting it. the red line is emission the blue line is excitation. i'm having trouble interpreting properly. just want to know if there is any evidence of raman or rayleigh peaks in the spectra.arrow_forward
- Give the major product of the following reaction. excess 1. OH, H₂O 1.OH H CH3CH2CH21 H 2. A.-H₂O Draw the molecule on the canvas by choosing buttons from the Tools (for bonds), Atoms, and Advanced Template toolbars. The single bond is active by default.arrow_forward2. Use Hess's law to calculate the AH (in kJ) for: rxn CIF(g) + F2(g) → CIF 3 (1) using the following information: 2CIF(g) + O2(g) → Cl₂O(g) + OF 2(g) AH = 167.5 kJ ΔΗ 2F2 (g) + O2(g) → 2 OF 2(g) 2C1F3 (1) + 202(g) → Cl₂O(g) + 3 OF 2(g) о = = -43.5 kJ AH = 394.1kJarrow_forwardci Draw the major product(s) of the following reactions: (3 pts) CH3 HNO3/H2SO4 HNO3/ H2SO4 OCH3 (1 pts)arrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning




