
Concept explainers
Devise efficient syntheses of each of the following compounds from the designated startingmaterials. You may also use any necessary organic or inorganic reagents.

Interpretation:
Efficient syntheses are to be designed for each of the five compounds with designated starting materials and necessary organic or inorganic compounds.
Concept introduction:
Synthesis of amines can be done in a variety of ways, based on forming a bond between a carbon and a nitrogen.
Nucleophilic substitution reactions on alkyl halides or alcohols by a nitrogen containing reagent is one of the common methods.
Primary amines can be synthesized by nucleophilic substitution of an alkyl halide with cyanide. The resulting compound, called a nitrile, yields a primary amine on catalytic reduction with hydrogen.
Secondary and particularly tertiary amines can be formed by reaction between ammonia and an excess of alkyl halide.
Another method involves nucleophilic addition of simpler amines to an aldehyde or a ketone.
Carboxylic acid derivatives such as acid halides, anhydrides, and esters undergo nucleophilic substitution by ammonia or a variety of simpler amines to give amides. The amides on reduction gives amines.
Benzyl radical is stabilized by delocalization of the unpaired electron on to the benzene ring. This helps in selective radical substitution of hydrogens and halogens in benzylic position.
Answer to Problem 45P
Solution:
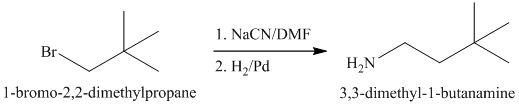
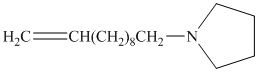 from
from
c)
 from
from 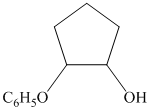 :
:
d)
e)
 from
from 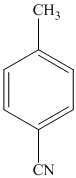 :
:
Explanation of Solution
a)
The substrate is a primary alkyl halide; therefore, substitution of the bromine requires a strong nucleophile such as cyanide ion via an
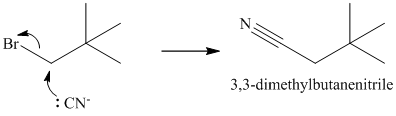
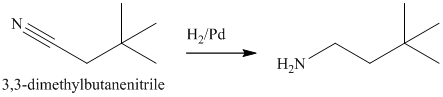
The nitrile yields the desired amine on catalytic reduction.
b)
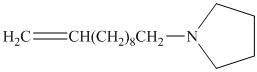 from
from
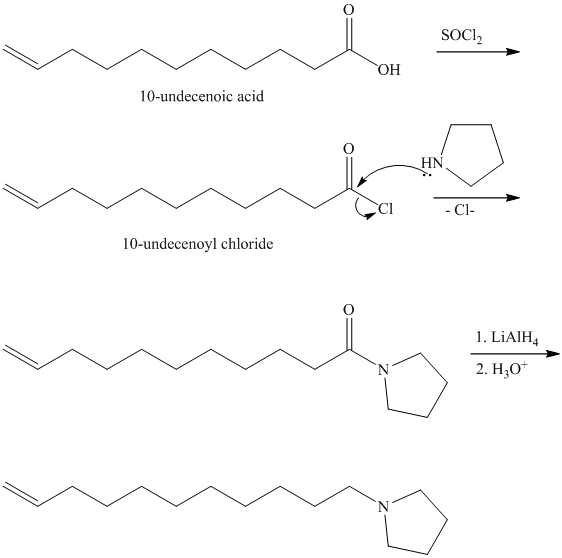
Carboxylic acids and amines can react to form amides by nucleophilic substitution of the hydroxyl group by the amine. However, carboxylic acids are poor electrophiles and are relatively unreactive. The first step is then to activate the acid by converting it to a stronger electrophile such as an acid chloride.
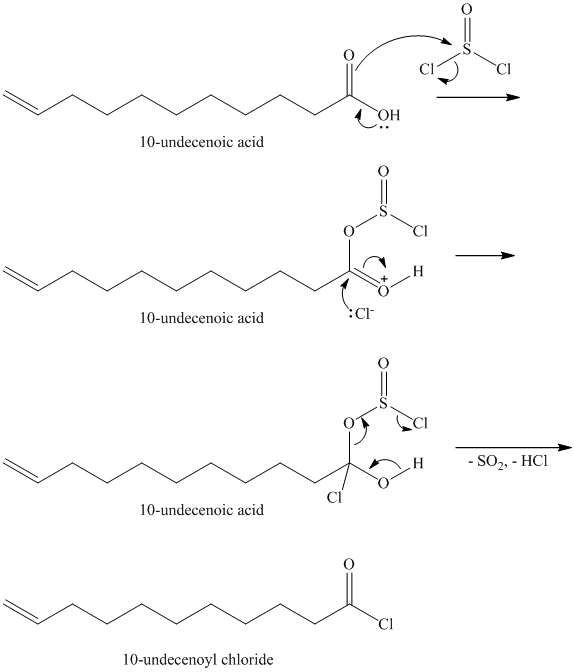
The chloride then undergoes substitution by
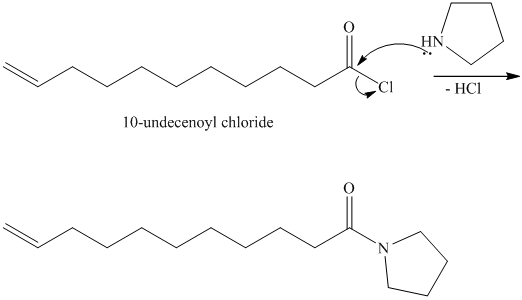
This contains both a carbonyl group that needs to be reduced to a methylene and a double bond. Catalytic reduction will result in reduction of the carbonyl group as well as an unwanted reduction of the double bond. Therefore, reduction of the carbonyl to methylene bridge is done with lithium aluminum hydride.
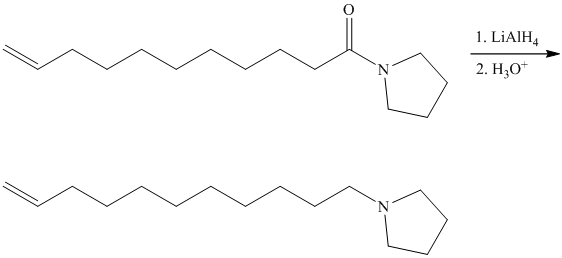
The hydride ion (
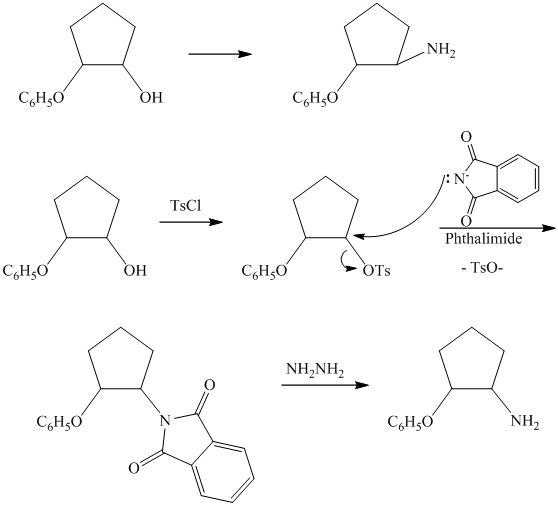
c)
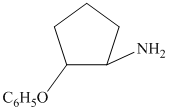 from
from 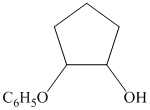 :
:
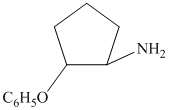
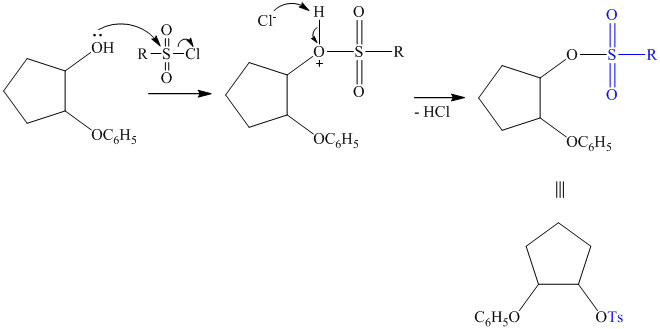
The synthesis requires substitution of the hydroxyl group of the substrate. Hydroxyl group is a poor leaving group as the hydroxide ion is a strong base. It first needs to be converted to a better leaving group. This is done by reaction with tosyl chloride, to give a tosylate.
Tosylate ion is the conjugate base of a strong acid, so it can be easily replaced by a suitable nucleophile like phthalimide.
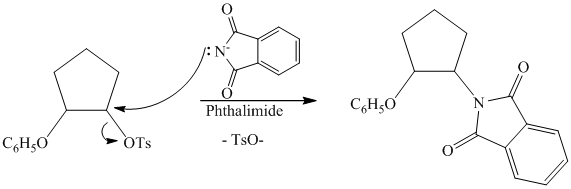
Reaction of the tosylate with phthalimide is an
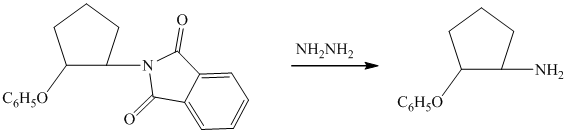
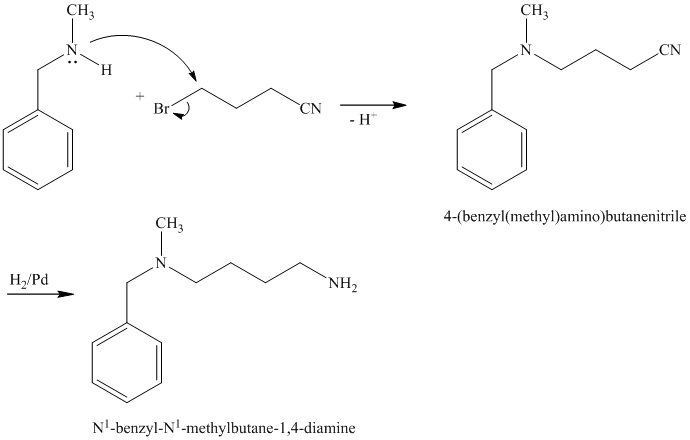
d)
The first of the designated starting compounds
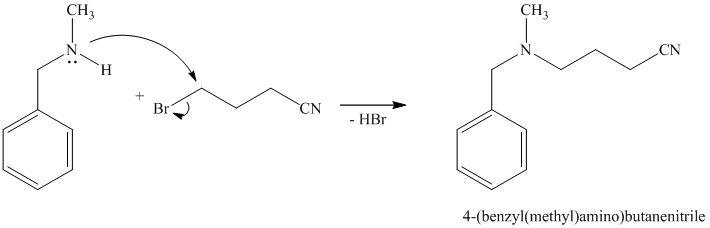
The nitrile (cyanide) function can then be reduced with hydrogen over palladium to yield the desired product.
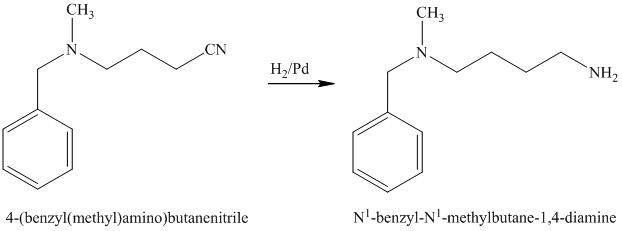
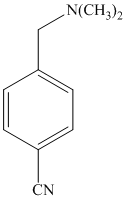
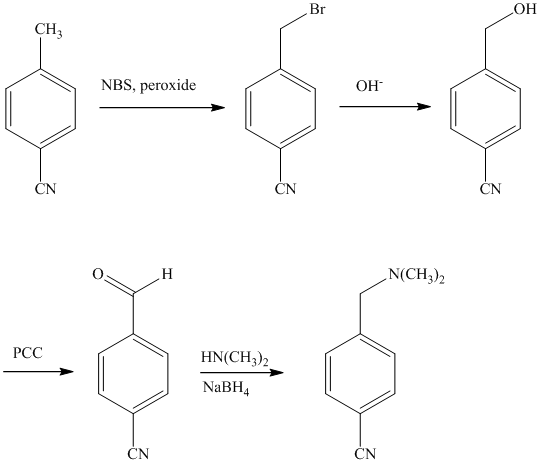
e)
The methyl substituent on the benzene ring needs to be converted to an amine. This requires first converting it to an alkyl halide. This is a substitution of a hydrogen in a benzylic position. A convenient method is to treat it with
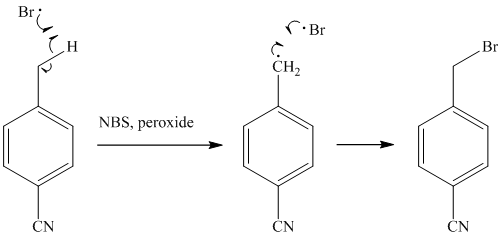
The resulting bromide can be reacted directly with dimethyl amine to yield the product. However, there is a possibility of formation of a quaternary ammonium compound. Therefore, the bromide is converted to a primary alcohol by reacting it with hydroxide.
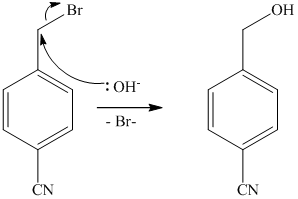
The alcohol is then oxidized to aldehyde using pyridiniumchlorochromate.
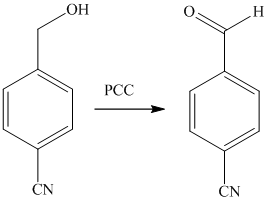
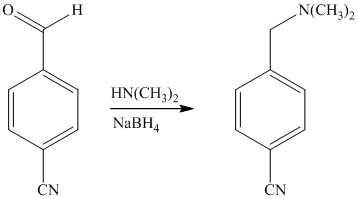
Reaction of the aldehyde can then be subjected to reductive amination, reaction with dimethyl amine in the presence of sodium borohydride, to yield the desired product. The reaction initially forms an imine, but that is directly reduced by sodium borohydrideinstead of isolating it.
Want to see more full solutions like this?
Chapter 22 Solutions
Organic Chemistry - Standalone book
- true or false The equilibrium constant for this reaction is 0.20. N2O4(g) ⇔ 2NO2(g) Based on the above, the equilibrium constant for the following reaction is 0.4. 2N2O4(g) ⇔ 4NO2(g)arrow_forwardtrue or false Using the following equilibrium, if heat is added the equilibrium will shift toward the reactants. N2(g) + 3H2(g) ⇔ 2NH3(g) + heatarrow_forwardTrue or False Using the following equilibrium, if heat is added the equilibrium will shift toward the products. N2O4(g) + heat ⇔ 2NO2(g)arrow_forward
- true or false Using the following equilibrium, if solid carbon is added the equilibrium will shift toward the products. C(s) + CO2(g) ⇔ 2CO(g)arrow_forwardProvide the complete mechanism for the reaction below. You must include appropriate arrows,intermediates, and formal charges. Please also provide a reason to explain why the 1,4-adduct is preferred over the 1,3-adduct.arrow_forwardWhich of the following pairs are resonance structures of one another? I. III. || III IV + II. :0: n P !༠ IV. EN: Narrow_forward
- Predict the major organic product(s) and byproducts (either organic or inorganic) for thefollowing reactions.arrow_forwardA 8.25 g sample of aluminum at 55°C released 2500 J of heat. The specific heat of aluminum is 0.900 J/g°C. The density of aluminum is 2.70 g/mL. Calculate the final temperature of the aluminum sample in °C.arrow_forwardPredict the major organic product(s) and byproducts (either organic or inorganic) for thefollowing reactions.arrow_forward
- Predict the major organic product(s) and byproducts (either organic or inorganic) for thefollowing reaction.arrow_forwardplease helparrow_forwardExperiment 1 Data Table 1: Conservation of Mass - Initial Mass Data Table 1 Data Table 2 Data Table 3 Data Table 4 Panel 1 Photo 1 Data Table 5 Reaction Mass of test tube and 5.0% HC₂H₂O2 (g) # (A) (B) Mass of NaHCO, (g) Mass of balloon and NaHCO, (g) (C) 0.10 1 0829 14.38g 0.20 2 0.929 14.29g 0.35 1.00g 3 14.25g 0.50 1.14g 14.29 Experiment 1 Data Table 2: Moles of HC2H3O2 Reaction Volume of Mass of Moles of HC₂H₂O₂ 5.0% Vinegar (g) (ML) 5.0 0.25 0042 mol 2 5.0 0.25 0042 mol 3 5.0 0.25 0042 mol 5.0 0.25 0042 mol Experiment 1 Data Table 3: Moles of NaHCO3 Reaction Mass of NaHCO (g) 10g 20g 35g 50g Experiment 1 Data Table 4: Theoretical Yield of CO₂ Reaction # 1 2 3 Experiment 1 Total mass before reaction (g) (D=A+C) 15.29 15.21g 15.25g 15.349 Exercise 1 Data Table 1 Data Table 2 Data Table 3 Data Table 4 Panel 1 Photo 1 Data Table 5 Exercise 1- Data Table 1 Data Table 2 DataTable 3 Data Table 4 Panel 1 Photo 1 Data Table 5 Exercise 1- Moles of NaHCO 0012 mol 0025 mol 0044 mol 0062 mol…arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

