
ORGANIC CHEMISTRY-PACKAGE >CUSTOM<
10th Edition
ISBN: 9781260028355
Author: Carey
Publisher: MCG CUSTOM
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 21.5, Problem 15P
Problem 21.15
Write the structure of the Dieckmann cyclization product formed on treatment of each of the following diesters with sodium ethoxide, followed by acidification.
(a) 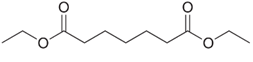 (c)
(c) 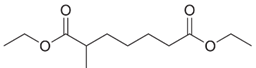
(b) 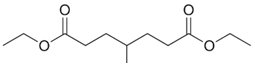
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
b)
Arrange the following compounds according to the reactivity towards nitration. Give reasons for
your choice.
CCH3
NHČCH,
CH3
16.48 D is an intermediate in the synthesis of rosiglitazone (trade name
Avandia), a drug used to treat type 2 diabetes. Suggest two different
methods to prepare the ether in D by substitution reactions.
CHO
D
omoss
rosiglitazone
H
How many different products are obtained upon ozonolysis of
?compound A
A) 1
B )2
C) 4
D) 3
Chapter 21 Solutions
ORGANIC CHEMISTRY-PACKAGE >CUSTOM<
Ch. 21.1 - Prob. 1PCh. 21.1 - Prob. 2PCh. 21.1 - Prob. 3PCh. 21.1 - Prob. 4PCh. 21.1 - Prob. 5PCh. 21.2 - Prob. 6PCh. 21.2 - Prob. 7PCh. 21.2 - Prob. 8PCh. 21.3 - Prob. 9PCh. 21.3 - Prob. 10P
Ch. 21.3 - Prob. 11PCh. 21.4 - Prob. 12PCh. 21.4 - Prob. 13PCh. 21.5 - Prob. 14PCh. 21.5 - Problem 21.15 Write the structure of the Dieckmann...Ch. 21.5 - Prob. 16PCh. 21.5 - Prob. 17PCh. 21.6 - Prob. 18PCh. 21.6 - Prob. 19PCh. 21.6 - Prob. 20PCh. 21.6 - Prob. 21PCh. 21.6 - Prob. 22PCh. 21.7 - Prob. 23PCh. 21.8 - Problem 21.24 Mesityl oxide is an industrial...Ch. 21.8 - Prob. 25PCh. 21.8 - Prob. 26PCh. 21.8 - Prob. 27PCh. 21.8 - Prob. 28PCh. 21 - Prob. 29PCh. 21 - Terreic acid, a naturally occurring antibiotic...Ch. 21 - Prob. 31PCh. 21 - Prob. 32PCh. 21 - Prob. 33PCh. 21 - Prob. 34PCh. 21 - Give the structure of the expected organic product...Ch. 21 - Prob. 36PCh. 21 - Prob. 37PCh. 21 - Prob. 38PCh. 21 - Prob. 39PCh. 21 - Give the structure of the principal organic...Ch. 21 - Prob. 41PCh. 21 - Prob. 42PCh. 21 - Prob. 43PCh. 21 - Prob. 44PCh. 21 - Prob. 45PCh. 21 - Prob. 46PCh. 21 - Prob. 47PCh. 21 - The use of epoxides as alkylating agents for...Ch. 21 - Prob. 49PCh. 21 - Show how you could prepare each of the following...Ch. 21 - Prob. 51PCh. 21 - Prob. 52PCh. 21 - Prob. 53PCh. 21 - Prob. 54PCh. 21 - The - methylene ketone sarkomycin has an...Ch. 21 - - Lactone can be prepared in good yield from...Ch. 21 - Prob. 57PCh. 21 - Prob. 58DSPCh. 21 - The Enolate Chemistry of Dianionss The synthetic...Ch. 21 - Prob. 60DSPCh. 21 - Prob. 61DSPCh. 21 - Prob. 62DSPCh. 21 - Prob. 63DSP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Answer ALL parts. (a) Treatment of enone C with tetrabutylammonium fluoride leads to the formation of heterocycle D. 2 steps BU4NF C9H1602 A + B D OSIME,tBu (i) Enone C can be prepared from alkyne A and aldehyde B in two steps via a gold-catalysed reaction. Identify A and B and provide reagents/conditions for a synthesis of C. (ii) Give the structure of D and classify the ring-closing reaction for its formation from C according to Baldwin's rules (iii) Sketch the key molecular orbitals involved in the ring-closing reaction in part (ii) and hence explain whether the reaction is favourable or unfavourable.arrow_forward8.30 Outline an efficient synthesis of each of the following compounds from the indicated starting material and any necessary organic or inorganic reagents: (j) (S)-CH3CH2CHCH3 from (R)-sec-butyl alcoholarrow_forwardPrepare the compound by using suitable reagents. More than one step will likely be required.arrow_forward
- A carbonyl compound on reaction with lodine in presence of sodium hydroxide followed by hydrolysis results in formation of iodoform and sodium salt of butanoic acid. Give the structure of the compound and reactions. A M A ::arrow_forward8.30 Outline an efficient synthesis of each of the following compounds from the indicated starting material and any necessary organic or inorganic reagents: (g) Isopropyl azide from isopropyl alcohol (h) Isopropyl azide from 1-propanol (i) (S)-sec-butyl azide from (R)-sec-butyl alcoholarrow_forwardWittig reaction is a synthetic method for the preparation of an alkene from a carbonyl compound using a phosphonium ylide. i) Propose the starting materials needed to synthesize alkene A by Wittig reaction. ii) Suggest two possible routes that lead to the formation of alkene A. Aarrow_forward
- 21.3 Provide the reagents. -ОН CIarrow_forwardThe analgesic naproxen can be prepared by a stepwise reaction sequence from ester A. Using enolate alkylation in one step, what reagents are needed to convert A to naproxen? Write the structure of each intermediate. Explain why a racemic product is formed.arrow_forward8.30 Outline an efficient synthesis of each of the following compounds from the indicated starting material and any necessary organic or inorganic reagents: (d) NCCH2CH2CN from ethyl alcohol (e) Isobutyl iodide from isobutyl chloride (f) Isobutyl iodine from tert-butyl chloridearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:9781305577190
Author:Kenneth L. Williamson, Katherine M. Masters
Publisher:Brooks Cole

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:9781305446021
Author:Lampman
Publisher:CENGAGE LEARNING - CONSIGNMENT


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Lipids - Fatty Acids, Triglycerides, Phospholipids, Terpenes, Waxes, Eicosanoids; Author: The Organic Chemistry Tutor;https://www.youtube.com/watch?v=7dmoH5dAvpY;License: Standard YouTube License, CC-BY