
EBK ORGANIC CHEMISTRY
8th Edition
ISBN: 8220102744127
Author: Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 2.10, Problem 43P
A naturally occurring amino acid such as alanine has a group that is a
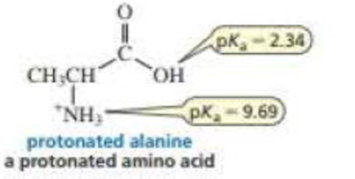
- a. If the pKa value of a carboxylic add such as acetic acid is about 5 (see Table 2.1 ), then why is the pKa value of the carboxylic acid group of alanine so much lower?
- b. Draw the structure of alanine in a solution at pH = 0.
- c. Draw the structure of alanine in a solution at physiological pH (pH 7.4).
- d. Draw the structure of alanine in a solution at pH = 12.
- e. Is there a pH at which alanine is uncharged (that is. neither group has a charge)?
- f. At what pH does alanine have no net charge (that is, the amount of negative charge is the same as the amount of positive charge)?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Some weak acids and their pKa values are given below. Which one of these acids will have the STRONGEST conjugate base?
Select one:
a. Phenol; pKa = 10.00
b. Methanoic acid; pKa = 3.75
c. Bromoethanoic acid; pKa = 2.9
d. Methanol; pKa = 15.54
What is the Kb of a base whose pKa is 8.9?
Given the following information determine which of these acids is the strongest?
Ka for boric acid is 7.3 x 10–10
pKa of acetic acid is 4.75
Ka for lactic acid is 8.4 x 10–4
pKa of phenol is 9.89
A
acetic acid
B
formic acid
C
lactic acid
D
phenol
Chapter 2 Solutions
EBK ORGANIC CHEMISTRY
Ch. 2.1 - Which of the following are not acids? CH3COOH CO2...Ch. 2.1 - Consider the following reaction: a. What is the...Ch. 2.1 - Draw the products of the addbase renc1 ion when a....Ch. 2.1 - a. What is the conjugate acid of each or the...Ch. 2.2 - a. Which is a stronger acid: one with a pKa of 5.2...Ch. 2.2 - An acid has a Ka of 4.53 106 in water. What is...Ch. 2.2 - Prob. 7PCh. 2.2 - Antacids are compounds that neutralize stomach...Ch. 2.2 - Are the following body fluids acidic or basic? a....Ch. 2.3 - Draw the conjugate acid of each of the following:...
Ch. 2.3 - a. Write an equation showing CH3OH reacting as an...Ch. 2.3 - Estimate the pKa values of the following...Ch. 2.3 - a. Which is a stronger base: CH3COO or HCOO? (The...Ch. 2.3 - Using the pKa values in Section 2.3, rank the...Ch. 2.4 - Prob. 15PCh. 2.5 - a. For each of the acid-base reactions in Section...Ch. 2.5 - Ethyne has a pKa value of 25, water has a pKa...Ch. 2.5 - Which of the following bases can remove a proton...Ch. 2.5 - Calculate the equilibrium constant for the...Ch. 2.6 - Rank the ions (CH3, NH2, HO, and F) from most...Ch. 2.6 - Rank the carbanions shown in the margin from most...Ch. 2.6 - Which is the stronger acid?Ch. 2.6 - Prob. 23PCh. 2.6 - What reaction in Problem 23 has the smallest...Ch. 2.6 - Rank the halide ions (F, Cl, Br, and l) from...Ch. 2.6 - a. Which is more electronegative, oxygen or...Ch. 2.6 - Which is a stronger acid? a. HCl or HBr b....Ch. 2.6 - a. Which of the halide ions (F, Cl, Br, and l) is...Ch. 2.6 - Which is a stronger base? (The potential maps in...Ch. 2.7 - What is a stronger acid? a. CH3OCH2CH2OH or...Ch. 2.7 - Rank the following compounds from strongest add to...Ch. 2.7 - What is a stronger base?Ch. 2.8 - For each of the following compounds, indicate the...Ch. 2.8 - Prob. 35PCh. 2.8 - Which is a stronger acid? Why?Ch. 2.8 - Fosamax (shown on the previous page) has six...Ch. 2.9 - Using the table of pKa values given in Appendix I,...Ch. 2.10 - For each of the following compounds (here shown in...Ch. 2.10 - As long as the pH is not less than _______, at...Ch. 2.10 - a. Indicate whether a protonated amine (RN+H3)...Ch. 2.10 - A naturally occurring amino acid such as alanine...Ch. 2.10 - a. At what pH is the concentration of a compound,...Ch. 2.10 - For each of the following compounds, indicate the...Ch. 2.10 - Given the data in Problem 47: a. What pH would you...Ch. 2.11 - Write the equation that shows how a buffer made by...Ch. 2.12 - Draw the products of the following react ions. Use...Ch. 2.12 - What product are formed when each of the following...Ch. 2 - Which is a stronger base? a. HS or HO b. CH3O or...Ch. 2 - According to the explanations by Lewis, if a...Ch. 2 - a. Rank the following alcohols from strongest to...Ch. 2 - a. Rank the following carboxylic acids from...Ch. 2 - Prob. 57PCh. 2 - For the following compound. a. draw its conjugate...Ch. 2 - Rank the following compounds from strongest to...Ch. 2 - Prob. 60PCh. 2 - Prob. 61PCh. 2 - a. Rank the following alcohols from strongest to...Ch. 2 - A single bond between two carbons with different...Ch. 2 - For each compound, indicate the atom that is most...Ch. 2 - a. Given the Ka values, estimate the pKa value of...Ch. 2 - Tenormin, a member of the group of drugs known as...Ch. 2 - From which of the following compounds can HO...Ch. 2 - a. For each of the following pairs of reactions,...Ch. 2 - Prob. 69PCh. 2 - Which is a stronger acid? a. b. c. d.Ch. 2 - Prob. 71PCh. 2 - Prob. 72PCh. 2 - Given that pH+ pOH = 14 and that the concentration...Ch. 2 - How could you separate a mixture of the following...Ch. 2 - Prob. 75PCh. 2 - a. If an add with a pKa of 5.3 is in an aqueous...Ch. 2 - Calculate the pH values of the following...Ch. 2 - Prob. 1PCh. 2 - Prob. 2PCh. 2 - Prob. 3PCh. 2 - Which of the reactions in Problem 3 favor...Ch. 2 - Prob. 5PCh. 2 - Prob. 6PCh. 2 - Prob. 7PCh. 2 - Which is the stronger acid? a. ClCH2CH2OH or...Ch. 2 - Prob. 9PCh. 2 - Prob. 10PCh. 2 - Which is a more stable base? Remembering that the...Ch. 2 - Which is the Stronger acid?Ch. 2 - Prob. 13PCh. 2 - a. Draw the structure of (CH3COOH (pKa = 4.7) at...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A naturally occurring amino acid such as alanine has a group that is a carboxylic acid and a group that is a protonated amine. The pKa values of the two groups are shown.(a). If the pKa value of a carboxylic acid such as acetic acid is about 5, then why is the pKa value of the carboxylic acid group of alanine so much lower? (b). Draw the structure of alanine in a solution at pH = 0. (c). Draw the structure of alanine in a solution at physiological pH (pH 7.4).(d). Draw the structure of alanine in a solution at pH = 12. (e). Is there a pH at which alanine is uncharged (that is, neither group has a charge)? (f) At what pH does alanine have no net charge (that is, the amount of negative charge is the same as the amount of positive charge)?arrow_forwarda) Calculate Ka of formic acid whose pKa= 3.75. b) Calculate pKa of nitromethane whose Ka= 5.0 x 10"arrow_forwardPlease see attached imagearrow_forward
- Which of the following acid/conjugate base pairs has a pKa value of 9.44? A. Ammonium/ammonia B. carboxylic acid/carboxylate C. imidazolium/imidazolearrow_forward11. Which conjugate base should be the strongest out of these choices? (pKa values of their respective acid is provided) A. NO₂ (3.15) B. CN (9.2) C. NH3 (9.26) D. NO3 (-1.5) E. HS (7.04)arrow_forwardThe pkb of methylamine, CH3NH2, is 3.36. Calculate the pka of its conjugate acid, CH3NH3*. pka =arrow_forward
- Consider the amino acid norvaline: O NH2 OH H C3 a. The pKa of the carboxylic acid group is 2.36. The pKa of the amino group is 9.76. Show how to calculate the isoelectric point, pI of this amino acid. b. Draw the structure of norvaline in a solution of NaOH (aq) c. Draw the structure of norvaline in a solution of H2SO4 (aq)arrow_forwardGiven a Ka value of 1.3 X 10-10 for phenol (a benzene with an alcohol group on it-aromatic alcohols are acidic-who knew?), what is its pKa? 7.20 8.75 6.80 9.89arrow_forwardWhich of the following acids is the strongest? A. Ascorbic acid, pK₂ = 11.3 B. Chlorous acid, pka = 1.96 C. Hypobromous acid, pK₂ = 8.64 D. Trifluoroacetic acid, pK₂ = 0.23 A OB D O Carrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Biomolecules - Protein - Amino acids; Author: Tutorials Point (India) Ltd.;https://www.youtube.com/watch?v=ySNVPDHJ0ek;License: Standard YouTube License, CC-BY