
Modified Mastering Physics without Pearson eText-- Instant Access -- for Physics for Scientists & Engineers with Modern Physics
5th Edition
ISBN: 9780134402659
Author: GIANCOLI, Douglas
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 21, Problem 37P
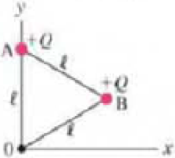
(II) (a) Determine the electric field
FIGURE 21–59 Problem 38.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
2. A stone is dropped into a pool of water causing ripple to spread out. After 10 s
the circumference of the ripple is 20 m. Calculate the velocity of the wave.
10. Imagine you have a system in which you have 54 grams of ice. You can melt this
ice and then vaporize it all at 0 C. The melting and vaporization are done reversibly
into a balloon held at a pressure of 0.250 bar. Here are some facts about water you
may wish to know. The density of liquid water at 0 C is 1 g/cm³. The density of ice at 0
C is 0.917 g/cm³. The enthalpy of vaporization of liquid water is 2.496 kJ/gram and the
enthalpy of fusion of solid water is 333.55 J/gram.
A. How much energy does the ice absorb as heat when it melts?
B. How much work is involved in melting the ice?
C. What is the total change in energy for melting the ice?
D. What is the enthalpy change for melting the ice?
E. What is the entropy change for melting the ice?
F. What is the change in Helmholtz energy for melting the ice?
G. What is the change in Gibbs energy for melting the ice?
In the figure Q = 5.7 nC and all other quantities are accurate to 2 significant figures. What is the magnitude of the force on the charge Q? (k = 1/4πε 0 = 8.99 × 109 N · m2/C2)
Chapter 21 Solutions
Modified Mastering Physics without Pearson eText-- Instant Access -- for Physics for Scientists & Engineers with Modern Physics
Ch. 21.5 - Return to the Chapter-Opening Question, page 559,...Ch. 21.5 - Prob. 1BECh. 21.5 - Determine the magnitude and direction of the net...Ch. 21.5 - (a) Consider two point charges of the same...Ch. 21.6 - Four charges of equal magnitude, but possibly...Ch. 21 - If you charge a pocket comb by rubbing it with a...Ch. 21 - Why does a shirt or blouse taken from a clothes...Ch. 21 - Explain why fog or rain droplets tend to form...Ch. 21 - A positively charged rod is brought close to a...Ch. 21 - Why does a plastic ruler that has been rubbed with...
Ch. 21 - Contrast the net charge on a conductor to the free...Ch. 21 - Figures 217 and 218 show how a charged rod placed...Ch. 21 - When an electroscope is charged, the two leaves...Ch. 21 - Prob. 9QCh. 21 - Prob. 10QCh. 21 - The form of Coulombs law is very similar to that...Ch. 21 - We are not normally aware of the gravitational or...Ch. 21 - What experimental observations mentioned in the...Ch. 21 - When a charged ruler attracts small pieces of...Ch. 21 - Explain why the test charges we use when measuring...Ch. 21 - When determining an electric field, must we use a...Ch. 21 - Draw the electric field lines surrounding two...Ch. 21 - Assume that the two opposite charges in Fig. 2134a...Ch. 21 - Consider the electric field at the three points...Ch. 21 - Why can electric field lines never cross?Ch. 21 - Prob. 21QCh. 21 - Given two point charges, Q and 2Q, a distance ...Ch. 21 - Suppose the ring of Fig. 2128 has a uniformly...Ch. 21 - Consider a small positive test charge located on...Ch. 21 - We wish to determine the electric field at a point...Ch. 21 - In what ways does the electron motion in Example...Ch. 21 - Explain why there can be a net force on an...Ch. 21 - Describe the motion of the dipole shown in Fig....Ch. 21 - Prob. 1MCQCh. 21 - Prob. 2MCQCh. 21 - Prob. 3MCQCh. 21 - Prob. 4MCQCh. 21 - Prob. 5MCQCh. 21 - Prob. 6MCQCh. 21 - Prob. 7MCQCh. 21 - Prob. 8MCQCh. 21 - Prob. 9MCQCh. 21 - Prob. 10MCQCh. 21 - Prob. 11MCQCh. 21 - Prob. 12MCQCh. 21 - (I) What is the magnitude of the electric force of...Ch. 21 - Prob. 2PCh. 21 - Prob. 3PCh. 21 - Prob. 4PCh. 21 - Prob. 5PCh. 21 - Prob. 6PCh. 21 - Prob. 7PCh. 21 - Prob. 8PCh. 21 - Prob. 9PCh. 21 - (II) Compare the electric force holding the...Ch. 21 - (II) Two positive point charges are a fixed...Ch. 21 - Prob. 12PCh. 21 - Prob. 13PCh. 21 - Prob. 14PCh. 21 - Prob. 15PCh. 21 - (II) Two negative and two positive point charges...Ch. 21 - Prob. 17PCh. 21 - Prob. 18PCh. 21 - Prob. 19PCh. 21 - Prob. 20PCh. 21 - (III) Two positive charges +Q are affixed rigidly...Ch. 21 - Prob. 22PCh. 21 - Prob. 23PCh. 21 - Prob. 24PCh. 21 - Prob. 25PCh. 21 - Prob. 26PCh. 21 - Prob. 27PCh. 21 - Prob. 28PCh. 21 - Prob. 29PCh. 21 - (II) A long uniformly charged thread (linear...Ch. 21 - Prob. 31PCh. 21 - Prob. 32PCh. 21 - Prob. 33PCh. 21 - (II) Determine the direction and magnitude of the...Ch. 21 - Prob. 35PCh. 21 - (II) A very thin line of charge lies along the x...Ch. 21 - (II) (a) Determine the electric field E at the...Ch. 21 - (II) Draw, approximately, the electric field lines...Ch. 21 - (II) Two parallel circular rings of radius R have...Ch. 21 - (II) You are given two unknown point charges, Q1...Ch. 21 - Prob. 41PCh. 21 - (II) (a) Two equal charges Q are positioned at...Ch. 21 - (II) At what position, x = xM, is the magnitude of...Ch. 21 - (II) The uniformly charged straight wire in...Ch. 21 - (II) Determine the direction and magnitude of the...Ch. 21 - (II) Use your result from Problem 46 to find the...Ch. 21 - (II) A thin rod bent into the shape of an arc of a...Ch. 21 - (III) Suppose a uniformly charged wire starts at...Ch. 21 - Prob. 50PCh. 21 - (III) A thin rod of length carries a total charge...Ch. 21 - (III) Uniform plane of charge. Charge is...Ch. 21 - Prob. 53PCh. 21 - Prob. 54PCh. 21 - Prob. 55PCh. 21 - Prob. 56PCh. 21 - Prob. 57PCh. 21 - (II) A positive charge q is placed at the center...Ch. 21 - (II) A dipole consists of charges +e and e...Ch. 21 - (II) The HCl molecule has a dipole moment of about...Ch. 21 - (II) An electric dipole, of dipole moment p and...Ch. 21 - (II) Suppose both charges in Fig. 2145 (for a...Ch. 21 - (III) Suppose a dipole p is placed in a nonuniform...Ch. 21 - Prob. 64PCh. 21 - Prob. 65PCh. 21 - How close must two electrons be if the electric...Ch. 21 - Prob. 67GPCh. 21 - A water droplet of radius 0.018 mm remains...Ch. 21 - Estimate the net force between the CO group and...Ch. 21 - Suppose that electrical attraction, rather than...Ch. 21 - In a simple model of the hydrogen atom, the...Ch. 21 - A positive point charge Q1 = 2.5 105 C is fixed...Ch. 21 - When clothes are removed from a dryer, a 40-g sock...Ch. 21 - Dry air will break down and generate a spark if...Ch. 21 - Prob. 76GPCh. 21 - Packing material made of pieces of foamed...Ch. 21 - One type of electric quadrupole consists of two...Ch. 21 - Suppose electrons enter a uniform electric field...Ch. 21 - Prob. 80GPCh. 21 - Three very large square planes of charge are...Ch. 21 - Prob. 82GPCh. 21 - Prob. 83GPCh. 21 - Prob. 84GPCh. 21 - Prob. 85GPCh. 21 - A one-dimensional row of positive ions, each with...Ch. 21 - Prob. 87GPCh. 21 - Prob. 88GPCh. 21 - Prob. 89GPCh. 21 - Prob. 90GPCh. 21 - Prob. 91GPCh. 21 - Prob. 92GP
Additional Science Textbook Solutions
Find more solutions based on key concepts
How Would the experiments result charge if oxygen (O2) were induced in the spark chamber?
Biology: Life on Earth with Physiology (11th Edition)
Q8. Perform the calculation to the correct number of significant figures.
a) 0.121
b) 0.12
c) 0.12131
d) 0.121...
Chemistry: A Molecular Approach (4th Edition)
Explain all answers clearly, with complete sentences and proper essay structure if needed. An asterisk (*) desi...
Cosmic Perspective Fundamentals
In the following diagram, the white spheres represent hydrogen atoms and the blue Sphere represent the nitrogen...
Chemistry: The Central Science (14th Edition)
Starting with 10 bacterial cells per milliliter in a sufficient amount of complete culture medium with a 1-hour...
Microbiology with Diseases by Body System (5th Edition)
Lewis structure for lonic compounds
35. Is each compound best represented by an ionic or a covalent Lewis stru...
Introductory Chemistry (6th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- Now add a fourth charged particle, particle 3, with positive charge q3, fixed in the yz-plane at (0,d2,d2). What is the net force F→ on particle 0 due solely to this charge? Express your answer (a vector) using k, q0, q3, d2, i^, j^, and k^. Include only the force caused by particle 3.arrow_forwardFor a tornadoes and hurricanes, which of the following is most critical? an alert a watch a warning a predictionarrow_forwardWhen a warm front advances up and over a cold front, what is it called? front inversion stationary front cold front occlusion warm front occlusionarrow_forward
- 1) Consider two positively charged particles, one of charge q0 (particle 0) fixed at the origin, and another of charge q1 (particle 1) fixed on the y-axis at (0,d1,0). What is the net force F→ on particle 0 due to particle 1? Express your answer (a vector) using any or all of k, q0, q1, d1, i^, j^, and k^. 2) Now add a third, negatively charged, particle, whose charge is −q2− (particle 2). Particle 2 fixed on the y-axis at position (0,d2,0). What is the new net force on particle 0, from particle 1 and particle 2? Express your answer (a vector) using any or all of k, q0, q1, q2, d1, d2, i^, j^, and k^. 3) Particle 0 experiences a repulsion from particle 1 and an attraction toward particle 2. For certain values of d1 and d2, the repulsion and attraction should balance each other, resulting in no net force. For what ratio d1/d2 is there no net force on particle 0? Express your answer in terms of any or all of the following variables: k, q0, q1, q2.arrow_forwardA 85 turn, 10.0 cm diameter coil rotates at an angular velocity of 8.00 rad/s in a 1.35 T field, starting with the normal of the plane of the coil perpendicular to the field. Assume that the positive max emf is reached first. (a) What (in V) is the peak emf? 7.17 V (b) At what time (in s) is the peak emf first reached? 0.196 S (c) At what time (in s) is the emf first at its most negative? 0.589 x s (d) What is the period (in s) of the AC voltage output? 0.785 Sarrow_forwardA bobsled starts at the top of a track as human runners sprint from rest and then jump into the sled. Assume they reach 40 km/h from rest after covering a distance of 50 m over flat ice. a. How much work do they do on themselves and the sled which they are pushing given the fact that there are two men of combined mass 185 kg and the sled with a mass of 200 kg? (If you haven't seen bobsledding, watch youtube to understand better what's going on.) b. After this start, the team races down the track and descends vertically by 200 m. At the finish line the sled crosses with a speed of 55 m/s. How much energy was lost to drag and friction along the way down after the men were in the sled?arrow_forward
- For what type of force is it not possible to define a potential energy expression?arrow_forward10. Imagine you have a system in which you have 54 grams of ice. You can melt this ice and then vaporize it all at 0 C. The melting and vaporization are done reversibly into a balloon held at a pressure of 0.250 bar. Here are some facts about water you may wish to know. The density of liquid water at 0 C is 1 g/cm³. The density of ice at 0 C is 0.917 g/cm³. The enthalpy of vaporization of liquid water is 2.496 kJ/gram and the enthalpy of fusion of solid water is 333.55 J/gram.arrow_forwardConsider 1 mole of supercooled water at -10°C. Calculate the entropy change of the water when the supercooled water freezes at -10°C and 1 atm. Useful data: Cp (ice) = 38 J mol-1 K-1 Cp (water) 75J mol −1 K -1 Afus H (0°C) 6026 J mol −1 Assume Cp (ice) and Cp (water) to be independent of temperature.arrow_forward
- The molar enthalpy of vaporization of benzene at its normal boiling point (80.09°C) is 30.72 kJ/mol. Assuming that AvapH and AvapS stay constant at their values at 80.09°C, calculate the value of AvapG at 75.0°C, 80.09°C, and 85.0°C. Hint: Remember that the liquid and vapor phases will be in equilibrium at the normal boiling point.arrow_forward3. The entropy of an ideal gas is S = Nkg In V. Entropy is a state function rather than a path function, and in this problem, you will show an example of the entropy change for an ideal gas being the same when you go between the same two states by two different pathways. A. Express ASV = S2 (V2) - S₁(V1), the change in entropy upon changing the volume from V₁to V2, at fixed particle number N and energy, U. B. Express ASN = S₂(N₂) - S₁ (N₁), the change in entropy upon changing the particle number from N₁ to N2, at fixed volume V and energy U. C. Write an expression for the entropy change, AS, for a two-step process (V₁, N₁) → (V2, N₁) → (V2, N₂) in which the volume changes first at fixed particle number, then the particle number changes at fixed volume. Again, assume energy is constant.arrow_forwardPlease don't use Chatgpt will upvote and give handwritten solutionarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Classical Dynamics of Particles and SystemsPhysicsISBN:9780534408961Author:Stephen T. Thornton, Jerry B. MarionPublisher:Cengage Learning
Classical Dynamics of Particles and SystemsPhysicsISBN:9780534408961Author:Stephen T. Thornton, Jerry B. MarionPublisher:Cengage Learning Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College
College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning


Classical Dynamics of Particles and Systems
Physics
ISBN:9780534408961
Author:Stephen T. Thornton, Jerry B. Marion
Publisher:Cengage Learning

Physics for Scientists and Engineers: Foundations...
Physics
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Cengage Learning

College Physics
Physics
ISBN:9781938168000
Author:Paul Peter Urone, Roger Hinrichs
Publisher:OpenStax College

Principles of Physics: A Calculus-Based Text
Physics
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers, Technology ...
Physics
ISBN:9781305116399
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning
Physics Capacitor & Capacitance part 7 (Parallel Plate capacitor) CBSE class 12; Author: LearnoHub - Class 11, 12;https://www.youtube.com/watch?v=JoW6UstbZ7Y;License: Standard YouTube License, CC-BY