
COLLEGE PHY2053 W/MODIFIED ACCESS>BI<
16th Edition
ISBN: 9781323515303
Author: Knight
Publisher: PEARSON C
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
thumb_up100%
Chapter 2, Problem 56GP
Actual velocity data for a lion pursuing prey are shown in Figure P2.52. Estimate:
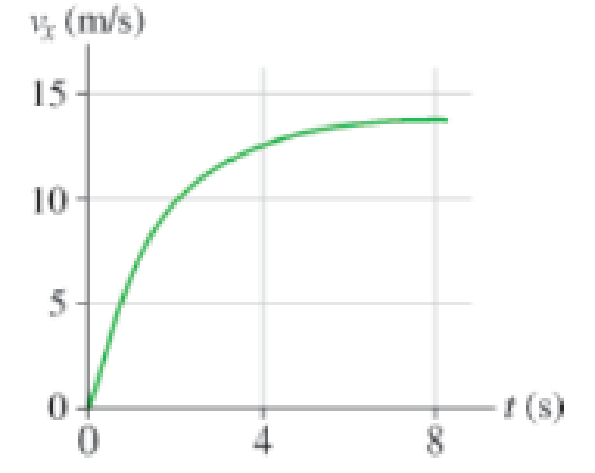
Figure P2.52
a. The initial acceleration of the lion.
b. The acceleration of the lion at 2 s and at 4 s.
c. The distance traveled by the lion between 0 s and 8 s.
Expert Solution & Answer
Trending nowThis is a popular solution!
Learn your wayIncludes step-by-step video

schedule05:55
Students have asked these similar questions
1)
Consider two positively charged particles, one of charge q0 (particle 0) fixed at the origin, and another of charge q1 (particle 1) fixed on the y-axis at (0,d1,0). What is the net force F→ on particle 0 due to particle 1?
Express your answer (a vector) using any or all of k, q0, q1, d1, i^, j^, and k^.
2)
Now add a third, negatively charged, particle, whose charge is −q2− (particle 2). Particle 2 fixed on the y-axis at position (0,d2,0). What is the new net force on particle 0, from particle 1 and particle 2?
Express your answer (a vector) using any or all of k, q0, q1, q2, d1, d2, i^, j^, and k^.
3)
Particle 0 experiences a repulsion from particle 1 and an attraction toward particle 2. For certain values of d1 and d2, the repulsion and attraction should balance each other, resulting in no net force. For what ratio d1/d2 is there no net force on particle 0?
Express your answer in terms of any or all of the following variables: k, q0, q1, q2.
A 85 turn, 10.0 cm diameter coil rotates at an angular velocity of 8.00 rad/s in a 1.35 T field, starting with the normal of the plane of the coil perpendicular to the field. Assume that the positive max emf is reached first.
(a) What (in V) is the peak emf?
7.17
V
(b) At what time (in s) is the peak emf first reached?
0.196
S
(c) At what time (in s) is the emf first at its most negative?
0.589
x s
(d) What is the period (in s) of the AC voltage output?
0.785
S
A bobsled starts at the top of a track as human runners sprint from rest and then jump into the sled. Assume they reach 40 km/h from rest after covering a distance of 50 m over flat ice. a. How much work do they do on themselves and the sled which they are pushing given the fact that there are two men of combined mass 185 kg and the sled with a mass of 200 kg? (If you haven't seen bobsledding, watch youtube to understand better what's going on.) b. After this start, the team races down the track and descends vertically by 200 m. At the finish line the sled crosses with a speed of 55 m/s. How much energy was lost to drag and friction along the way down after the men were in the sled?
Chapter 2 Solutions
COLLEGE PHY2053 W/MODIFIED ACCESS>BI<
Ch. 2 - A person gets in an elevator on the ground floor...Ch. 2 - a. Give an example of a vertical motion with a...Ch. 2 - Figure Q2.3 shows growth rings in the trunk of a...Ch. 2 - Sketch a velocity-versus-time graph for a rock...Ch. 2 - You are driving down the road at a constant speed....Ch. 2 - Prob. 6CQCh. 2 - Prob. 7CQCh. 2 - A ball is thrown straight up into the air. At each...Ch. 2 - Prob. 9CQCh. 2 - Figure Q2.10 shows an object's...
Ch. 2 - Figure Q2.11 shows the position graph for an...Ch. 2 - Figure Q2.12 shows the position-versus-time graphs...Ch. 2 - Figure Q2.13 shows a position-versus-time graph....Ch. 2 - Figure Q2.14 is the velocity-versus-time graph for...Ch. 2 - Figure Q2.15 shows the position graph of a car...Ch. 2 - Figure Q2.16 shows the position graph of a car...Ch. 2 - Figure Q2.17 shows an object's...Ch. 2 - The following options describe the motion of four...Ch. 2 - A car is traveling at Vx = 20 m/s. The driver...Ch. 2 - Velocity-versus-time graphs for three drag racers...Ch. 2 - Which of the three drag racers in Question 20 had...Ch. 2 - Chris is holding two softballs while standing on a...Ch. 2 - Suppose a plane accelerates from rest for 30 s,...Ch. 2 - Figure Q2.24 shows a motion diagram with the clock...Ch. 2 - Prob. 25MCQCh. 2 - Prob. 26MCQCh. 2 - Prob. 27MCQCh. 2 - Prob. 28MCQCh. 2 - Figure P2.1 shows a motion diagram of a car...Ch. 2 - For each motion diagram in Figure P2.2, determine...Ch. 2 - The position graph of Figure P2.3 shows a dog...Ch. 2 - A rural mail carrier is driving slowly, putting...Ch. 2 - For the velocity-versus-time graph of Figure P2.5:...Ch. 2 - Prob. 7PCh. 2 - A bicyclist has the position-versus-time graph...Ch. 2 - In major league baseball, the pitcher's mound is...Ch. 2 - In college softball, the distance from the...Ch. 2 - Alan leaves Los Angeles at 8:00am to drive to San...Ch. 2 - Richard is driving home to visit his parents. 125...Ch. 2 - In a 5.00 km race, one runner runs at a steady...Ch. 2 - In an 8.00 km race, one runner runs at a steady...Ch. 2 - Prob. 15PCh. 2 - While running a marathon, a long-distance runner...Ch. 2 - Prob. 17PCh. 2 - Prob. 18PCh. 2 - Prob. 19PCh. 2 - Prob. 20PCh. 2 - Prob. 21PCh. 2 - Prob. 22PCh. 2 - Prob. 23PCh. 2 - Prob. 24PCh. 2 - Prob. 25PCh. 2 - Small frogs that are good jumpers are capable of...Ch. 2 - A Thomson's gazelle can reach a speed of 13 m/s in...Ch. 2 - When striking, the pike, a predatory fish, can...Ch. 2 - a. What constant acceleration, in SI units, must a...Ch. 2 - When jumping, a flea rapidly extends its legs,...Ch. 2 - Prob. 31PCh. 2 - Light-rail passenger trains that provide...Ch. 2 - A cross-country skier is skiing along at a zippy...Ch. 2 - A small propeller airplane can comfortably achieve...Ch. 2 - Formula One racers speed up much more quickly than...Ch. 2 - Prob. 36PCh. 2 - A driver has a reaction time of 0.50 s, and the...Ch. 2 - Chameleons catch insects with their tongues, which...Ch. 2 - You're driving down the highway late one night at...Ch. 2 - Prob. 40PCh. 2 - A car is traveling at a steady 80 km/h in a 50...Ch. 2 - Prob. 42PCh. 2 - A simple model for a person running the 100m dash...Ch. 2 - Here's an interesting challenge you can give to a...Ch. 2 - In the preceding problem we saw that a person's...Ch. 2 - A gannet is a seabird that fishes by diving from a...Ch. 2 - Prob. 48PCh. 2 - Prob. 49PCh. 2 - A student at the top of a building of height h...Ch. 2 - Excellent human jumpers can leap straight up to a...Ch. 2 - A football is kicked straight up into the air; it...Ch. 2 - In an action movie, the villain is rescued from...Ch. 2 - Spud Webb was, at 5 ft 8 in, one of the shortest...Ch. 2 - A rock climber stands on top of a 50-m-high cliff...Ch. 2 - Actual velocity data for a lion pursuing prey are...Ch. 2 - A truck driver has a shipment of apples to deliver...Ch. 2 - Prob. 58GPCh. 2 - Prob. 60GPCh. 2 - The takeoff speed for an Airbus A320 jetliner is...Ch. 2 - Does a real automobile have constant acceleration?...Ch. 2 - Prob. 63GPCh. 2 - You are driving to the grocery store at 20 m/s....Ch. 2 - When you blink your eye, the upper lid goes from...Ch. 2 - A bush baby, an African primate, is capable of a...Ch. 2 - When jumping, a flea reaches a takeoff speed of...Ch. 2 - Certain insects can achieve seemingly impossible...Ch. 2 - A student standing on the ground throws a ball...Ch. 2 - A rock is tossed straight up with a speed of 20...Ch. 2 - Prob. 72GPCh. 2 - A car starts from rest at a stop sign. It...Ch. 2 - Heather and Jerry are standing on a bridge 50 m...Ch. 2 - A Thomson's gazelle can run at very high speeds,...Ch. 2 - We've seen that a man's higher initial...Ch. 2 - A pole-vaulter is nearly motionless as he clears...Ch. 2 - A Porsche challenges a Honda to a 400 m race....Ch. 2 - The minimum stopping distance for a car traveling...Ch. 2 - A rocket is launched straight up with constant...Ch. 2 - Free Fall on Different Worlds Objects in free fall...Ch. 2 - Free Fall on Different Worlds Objects in free fall...Ch. 2 - Free Fall on Different Worlds Objects in free fall...
Additional Science Textbook Solutions
Find more solutions based on key concepts
What dipeptides would be formed by heating a mixture of valine and N-protected leucine?
Organic Chemistry (8th Edition)
1. Which is a function of the skeletal system? (a) support, (b) hematopoietic site, (c) storage, (d) providing ...
Anatomy & Physiology (6th Edition)
How Would the experiments result charge if oxygen (O2) were induced in the spark chamber?
Biology: Life on Earth with Physiology (11th Edition)
2. What are the primary functions of the skeletal system?
Human Anatomy & Physiology (2nd Edition)
Why is it unlikely that two neighboring water molecules would be arranged like this?
Campbell Biology (11th Edition)
Modified True/False 9. A giant bacterium that is large enough to be seen without a microscope is Selenomonas.
Microbiology with Diseases by Body System (5th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- For what type of force is it not possible to define a potential energy expression?arrow_forward10. Imagine you have a system in which you have 54 grams of ice. You can melt this ice and then vaporize it all at 0 C. The melting and vaporization are done reversibly into a balloon held at a pressure of 0.250 bar. Here are some facts about water you may wish to know. The density of liquid water at 0 C is 1 g/cm³. The density of ice at 0 C is 0.917 g/cm³. The enthalpy of vaporization of liquid water is 2.496 kJ/gram and the enthalpy of fusion of solid water is 333.55 J/gram.arrow_forwardConsider 1 mole of supercooled water at -10°C. Calculate the entropy change of the water when the supercooled water freezes at -10°C and 1 atm. Useful data: Cp (ice) = 38 J mol-1 K-1 Cp (water) 75J mol −1 K -1 Afus H (0°C) 6026 J mol −1 Assume Cp (ice) and Cp (water) to be independent of temperature.arrow_forward
- The molar enthalpy of vaporization of benzene at its normal boiling point (80.09°C) is 30.72 kJ/mol. Assuming that AvapH and AvapS stay constant at their values at 80.09°C, calculate the value of AvapG at 75.0°C, 80.09°C, and 85.0°C. Hint: Remember that the liquid and vapor phases will be in equilibrium at the normal boiling point.arrow_forward3. The entropy of an ideal gas is S = Nkg In V. Entropy is a state function rather than a path function, and in this problem, you will show an example of the entropy change for an ideal gas being the same when you go between the same two states by two different pathways. A. Express ASV = S2 (V2) - S₁(V1), the change in entropy upon changing the volume from V₁to V2, at fixed particle number N and energy, U. B. Express ASN = S₂(N₂) - S₁ (N₁), the change in entropy upon changing the particle number from N₁ to N2, at fixed volume V and energy U. C. Write an expression for the entropy change, AS, for a two-step process (V₁, N₁) → (V2, N₁) → (V2, N₂) in which the volume changes first at fixed particle number, then the particle number changes at fixed volume. Again, assume energy is constant.arrow_forwardPlease don't use Chatgpt will upvote and give handwritten solutionarrow_forward
- 6. We used the constant volume heat capacity, Cv, when we talked about thermodynamic cycles. It acts as a proportionality constant between energy and temperature: dU = C₁dT. You can also define a heat capacity for constant pressure processes, Cp. You can think of enthalpy playing a similar role to energy, but for constant pressure processes δαρ C = (37) - Sup Ср ат P = ат Starting from the definition of enthalpy, H = U + PV, find the relationship between Cy and Cp for an ideal gas.arrow_forwardPure membranes of dipalmitoyl lecithin phospholipids are models of biological membranes. They melt = 41°C. Reversible melting experiments indicate that at Tm AHm=37.7 kJ mol-1. Calculate: A. The entropy of melting, ASm- B. The Gibbs free energy of melting, AGm- C. Does the membrane become more or less ordered upon melting? D. There are 32 rotatable CH2 CH2 bonds in each molecule that can rotate more freely if the membrane melts. What is the increase in multiplicity on melting a mole of bonds?arrow_forward5. Heat capacity often has a temperature dependence for real molecules, particularly if you go over a large temperature range. The heat capacity for liquid n-butane can be fit to the equation Cp(T) = a + bT where a = 100 J K₁₁ mol¹ and b = 0.1067 J K² mol¹ from its freezing point (T = 140 K) to its boiling point (T₁ = 270 K). A. Compute AH for heating butane from 170 K to 270 K. B. Compute AS for the same temperature range.arrow_forward
- 4. How much energy must be transferred as heat to cause the quasi-static isothermal expansion of one mole of an ideal gas at 300 K from PA = 1 bar to PB = 0.5 bar? A. What is VA? B. What is VB? C. What is AU for the process? D. What is AH for the process? E. What is AS for the process?arrow_forward1. The diagram shows the tube used in the Thomson experiment. a. State the KE of the electrons. b. Draw the path of the electron beam in the gravitational field of the earth. C. If the electric field directed upwards, deduce the direction of the magnetic field so it would be possible to balance the forces. electron gun 1KVarrow_forwardas a hiker in glacier national park, you need to keep the bears from getting at your food supply. You find a campground that is near an outcropping of ice. Part of the outcropping forms a feta=51.5* slopeup that leads to a verticle cliff. You decide that this is an idea place to hang your food supply out of bear reach. You put all of your food into a burlap sack, tie a rope to the sack, and then tie a bag full of rocks to the other end of the rope to act as an anchor. You currently have 18.5 kg of food left for the rest of your trip, so you put 18.5 kg of rocks in the anchor bag to balance it out. what happens when you lower the food bag over the edge and let go of the anchor bag? Determine the acceleration magnitude a of the two-bag system when you let go of the anchor bag?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College
College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning University Physics Volume 1PhysicsISBN:9781938168277Author:William Moebs, Samuel J. Ling, Jeff SannyPublisher:OpenStax - Rice University
University Physics Volume 1PhysicsISBN:9781938168277Author:William Moebs, Samuel J. Ling, Jeff SannyPublisher:OpenStax - Rice University College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

College Physics
Physics
ISBN:9781938168000
Author:Paul Peter Urone, Roger Hinrichs
Publisher:OpenStax College

College Physics
Physics
ISBN:9781285737027
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

Physics for Scientists and Engineers with Modern ...
Physics
ISBN:9781337553292
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

University Physics Volume 1
Physics
ISBN:9781938168277
Author:William Moebs, Samuel J. Ling, Jeff Sanny
Publisher:OpenStax - Rice University

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning
Position/Velocity/Acceleration Part 1: Definitions; Author: Professor Dave explains;https://www.youtube.com/watch?v=4dCrkp8qgLU;License: Standard YouTube License, CC-BY