
Concept explainers
Write a balanced chemical equation for the combustion of each of the following compounds:
a) Decane
b) Cyclodecane
c) Methylcyclononane
d) Cyclopentylcyclopentane
Interpretation:
A balanced chemical equation for the combustion of each of the given compounds is to be written.
Concept introduction:
Alkanes are inert in acid-base reactions but undergo oxidation-reduction reactions.
During combustion, alkanes undergo oxidation.
This combustion reaction of alkanes is exothermic, and the products formed are carbon dioxide and water.
When balancing the reaction, the carbon and hydrogen is balanced first, leaving oxygen for the last to balance.
Answer to Problem 39P
Solution:
a)
b)
c)
d)
Explanation of Solution
a) Decane
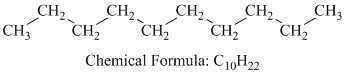
Decane is a straight chain alkane having the molecular formula
The combustion reaction of decane is written as follows:
To balance this reaction, first, the C and H are balanced.
Oxygen is balanced as follows:
The coefficients are converted to whole numbers as follows:
This is the complete balanced reaction of decane.
b) Cyclodecane
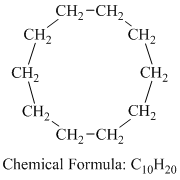
Cyclodecane is a cyclic alkane having the molecular formula
The combustion reaction of cyclodecane is written as follows:
To balance this reaction, first, the C and H are balanced.
Oxygen is balanced as follows:
This is the complete balanced reaction of cyclodecane.
c) Methylcyclononane
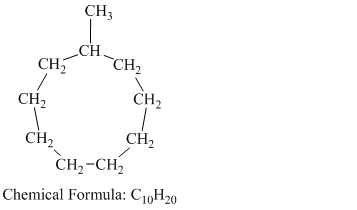
Methylcyclononane is a branched chain alkane having the molecular formula
The combustion reaction of methylcyclononane is written as follows:
To balance this reaction, first, the C and H are balanced.
Oxygen is balanced as follows:
This is the complete balanced reaction of methylcyclononane.
d) Cyclopentylcyclopentane
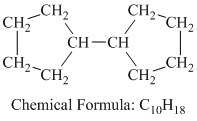
Cyclopentylcyclopentane is a branched chain alkane having the molecular formula
The combustion reaction of Cyclopentylcyclopentane is written as follows:
To balance this reaction, first, the C and H balanced.
Oxygen is balanced as follows:
The coefficients are converted to whole numbers as follows:
This is the complete balanced reaction of cyclopentylcyclopentane.
Want to see more full solutions like this?
Chapter 2 Solutions
ORGANIC CHEMISTRY-W/STUD.SOLN.MAN.
- option choice: Isoleucine Histidine Threonine Alanine Lysine Aspartate Tryptophan Tyrosine Leucine Arginine Cysteine Asparagine Valine Glutamine Glycine Methionine Serine Proline Phenylalanine Glutamatearrow_forwardsketch the nature of the metal-alkylidene bonding interactions.arrow_forwardPart C The perspective formula of isoleucine, an amino acid, is provided below. HOOC H₂NIC H 川 CH3 CH,CH3 Draw the Newman projection in staggered conformation for isoleucine by viewing the molecule along the C-2-C-3 bond. 1. Edit the Newman projection on the canvas. 2. Replace the appropriate hydrogens with the appropriate -CH3 or other groups. 3. If you need to start over, Undo or choose a Newman projection from the Templates toolbar (bottom). Important: Never delete the hydrogen atoms or bonds directly attached to the template, and do not move them by dragging or dropping them. That will break the projections structures. Only replace them! ▸ View Available Hint(s) 0 2 H± 3D EXP. L ד י CONT. 2 H 0 N оarrow_forward
 Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co





