
Physics (5th Edition)
5th Edition
ISBN: 9780321976444
Author: James S. Walker
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 17.4, Problem 4EYU
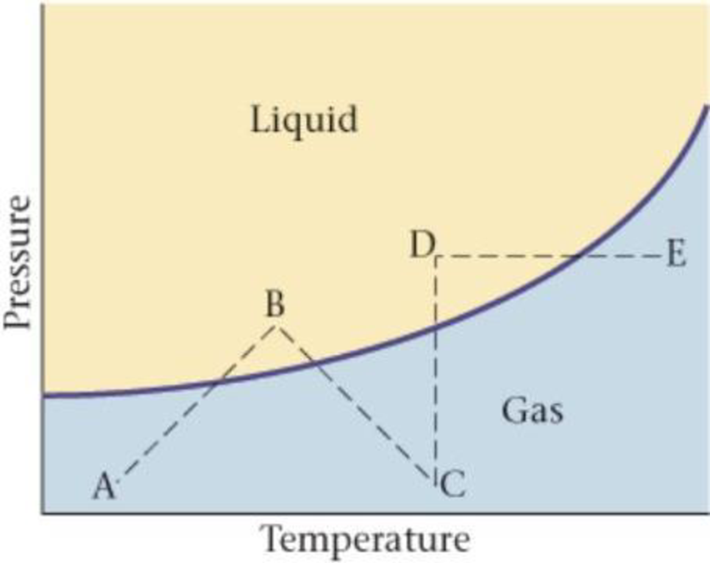
A portion of a substance’s phase diagram is shown in Figure 17-28. (a) Is the curve in the figure the fusion curve, the vapor-pressure curve, or the sublimation curve? (b) Which of the processes (AB, BC, CD, DE) corresponds to heating the substance at constant pressure? (c) Which of the processes (AB; BC; CD; DE) corresponds to increasing the pressure of the substance at constant temperature?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Find the current in 5.00 and 7.00 Ω resistors. Please explain all reasoning
Find the amplitude, wavelength, period, and the speed of the wave.
A long solenoid of length 6.70 × 10-2 m and cross-sectional area 5.0 × 10-5 m² contains
6500 turns per meter of length. Determine the emf induced in the solenoid when the
current in the solenoid changes from 0 to 1.5 A during the time interval from 0 to 0.20 s.
Number
Units
Chapter 17 Solutions
Physics (5th Edition)
Ch. 17.1 - Rank the following ideal-gas systems in order of...Ch. 17.2 - If the Kelvin temperature of a gas is doubled, by...Ch. 17.3 - A metal rod of a given initial length and...Ch. 17.4 - A portion of a substances phase diagram is shown...Ch. 17.5 - Which requires more heat: melting 100 kg of copper...Ch. 17.6 - An ice cube is placed in a cup of water. A few...Ch. 17 - How is the air pressure in a tightly sealed house...Ch. 17 - The average speed of air molecules in your room is...Ch. 17 - Is it possible to change both the pressure and the...Ch. 17 - Prob. 4CQ
Ch. 17 - A camping stove just barely boils water on a...Ch. 17 - An autoclave is a device used to sterilize medical...Ch. 17 - As the temperature of ice is increased, it changes...Ch. 17 - BIO Isopropyl alcohol is sometimes rubbed onto a...Ch. 17 - A drop of water on a kitchen counter evaporates in...Ch. 17 - (a) Is the number of molecules in one mole of N2...Ch. 17 - Predict/Explain If you put a helium-filled balloon...Ch. 17 - Two containers hold ideal gases at the same...Ch. 17 - Prob. 4PCECh. 17 - BIO After emptying her lungs, a person inhales 4.3...Ch. 17 - An automobile tire has a volume of 0.0185 m3. At a...Ch. 17 - Prob. 7PCECh. 17 - A compressed-air tank holds 0.500 m3 of air at a...Ch. 17 - Four ideal gases have the following pressures, P,...Ch. 17 - A balloon contains 3.9 liters of nitrogen gas at a...Ch. 17 - Prob. 11PCECh. 17 - Predict/Calculate A bicycle tire with a volume of...Ch. 17 - A 515-cm3 flask contains 0.460 g of a gas at a...Ch. 17 - Prob. 14PCECh. 17 - The air inside a hot-air balloon has an average...Ch. 17 - Prob. 16PCECh. 17 - Consider the system described in the previous...Ch. 17 - Prob. 18PCECh. 17 - Prob. 19PCECh. 17 - If the translational speed of molecules in an...Ch. 17 - At what temperature is the rms speed of H2 equal...Ch. 17 - Suppose a planet has an atmosphere of pure ammonia...Ch. 17 - Prob. 23PCECh. 17 - Prob. 24PCECh. 17 - Prob. 25PCECh. 17 - What is the temperature of a gas of CO2 molecules...Ch. 17 - The rms speed of a sample of gas is increased by...Ch. 17 - Prob. 28PCECh. 17 - A 380-mL spherical flask contains 0.065 mol of an...Ch. 17 - Prob. 30PCECh. 17 - A rock climber hangs freely from a nylon rope that...Ch. 17 - BIO To stretch a relaxed biceps muscle 2.5 cm...Ch. 17 - A 22-kg chimpanzee hangs from the end of a...Ch. 17 - The Marianas Trench The deepest place in all the...Ch. 17 - Four cylindrical rods with various cross-sectional...Ch. 17 - Predict/Calculate A steel wire 4.1 m long...Ch. 17 - BIO Spiderweb An orb weaver spider with a mass of...Ch. 17 - Predict/Calculate Two rods of equal length (0.55...Ch. 17 - A piano wire 0.82 m long and 0.93 mm in diameter...Ch. 17 - The formation of ice from water is accompanied by...Ch. 17 - Vapor Pressure for Water Figure 17-35 shows a...Ch. 17 - Using the vapor-pressure curve given in Figure...Ch. 17 - Prob. 43PCECh. 17 - Prob. 44PCECh. 17 - Predict/Calculate The Vapor Pressure of CO2 A...Ch. 17 - Phase Diagram for Water The phase diagram for...Ch. 17 - Phase Diagram for CO2 The phase diagram for CO2 is...Ch. 17 - Prob. 48PCECh. 17 - How much heat must be removed from 1.96 kg of...Ch. 17 - A heat transfer of 9.5 105 J is required to...Ch. 17 - How much heat must be added to 2.55 kg of copper...Ch. 17 - An ammonia refrigeration cycle involves the...Ch. 17 - Prob. 53PCECh. 17 - Prob. 54PCECh. 17 - Prob. 55PCECh. 17 - Figure 17-30 shows a temperature-versus-heat plot...Ch. 17 - Predict/Calculate Suppose the 1.000 kg of water in...Ch. 17 - Prob. 58PCECh. 17 - When you go out to your car one cold winter...Ch. 17 - A large punch bowl holds 3.99 kg of lemonade...Ch. 17 - A 155-g aluminum cylinder is removed from a liquid...Ch. 17 - An 825-g iron block is heated to 352 C and placed...Ch. 17 - Party Planning You are expecting to serve 32 cups...Ch. 17 - Predict/Calculate A 35-g ice cube at 0.0 C is...Ch. 17 - A 48-g block of copper at 12 C is added to 110 g...Ch. 17 - A 0 075-kg ice cube at 0.0 C is dropped into a...Ch. 17 - To help keep her barn warm on cold days, a farmer...Ch. 17 - CE As you go up in attitude, do you expect the...Ch. 17 - Prob. 69GPCh. 17 - Prob. 70GPCh. 17 - Prob. 71GPCh. 17 - Cooling Computers Researchers are developing heat...Ch. 17 - Prob. 73GPCh. 17 - Prob. 74GPCh. 17 - Evaporating Atmosphere Hydrogen gas evaporates...Ch. 17 - Prob. 76GPCh. 17 - A Boiling Geyser (a) The column of water that...Ch. 17 - A Melting Glacier (a) A glacier is made of ice of...Ch. 17 - Peter catches a 4 2-kg striped bass on a fishing...Ch. 17 - A steel ball (density=7860kg/m3) with a diameter...Ch. 17 - A lead brick with the dimensions shown in Figure...Ch. 17 - (a) Find the amount of heat that must be extracted...Ch. 17 - Mighty Ice Lift A tremendous force is generated...Ch. 17 - Orthopedic Implants Metals such as titanium and...Ch. 17 - Students on a spring break picnic bring a cooler...Ch. 17 - A 5.9-kg block of ice at 1.5 C slides on a...Ch. 17 - A cylindrical copper rod 37 cm long and 7.5 cm in...Ch. 17 - Prob. 88PPCh. 17 - Prob. 89PPCh. 17 - Prob. 90PPCh. 17 - Prob. 91PPCh. 17 - Referring to Example 17-17 (a) Find the final...Ch. 17 - Referring to Example 17-17 (a) Find the final...
Additional Science Textbook Solutions
Find more solutions based on key concepts
If someone at the other end of a room smokes a cigarette, you may breathe in some smoke. The movement of smoke ...
Campbell Essential Biology with Physiology (5th Edition)
18. SCIENTIFIC THINKING By measuring the fossil remains of Homo floresiensis, scientists have estimated its wei...
Campbell Biology: Concepts & Connections (9th Edition)
Which type of cartilage is most plentiful in the adult body?
Anatomy & Physiology (6th Edition)
Explain all answers clearly, with complete sentences and proper essay structure if needed. An asterisk (*) desi...
Cosmic Perspective Fundamentals
Flask A contains yeast cells in glucose-minimal salts broth incubated at 30C with aeration. Flask B contains ye...
Microbiology: An Introduction
All of the following processes are involved in the carbon cycle except: a. photosynthesis b. cell respiration c...
Human Biology: Concepts and Current Issues (8th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- A coat hanger of mass m = 0.255 kg oscillates on a peg as a physical pendulum as shown in the figure below. The distance from the pivot to the center of mass of the coat hanger is d = 18.0 cm and the period of the motion is T = 1.37 s. Find the moment of inertia of the coat hanger about the pivot.arrow_forwardReview Conceptual Example 3 and the drawing as an aid in solving this problem. A conducting rod slides down between two frictionless vertical copper tracks at a constant speed of 3.9 m/s perpendicular to a 0.49-T magnetic field. The resistance of th rod and tracks is negligible. The rod maintains electrical contact with the tracks at all times and has a length of 1.4 m. A 1.1-Q resistor is attached between the tops of the tracks. (a) What is the mass of the rod? (b) Find the change in the gravitational potentia energy that occurs in a time of 0.26 s. (c) Find the electrical energy dissipated in the resistor in 0.26 s.arrow_forwardA camera lens used for taking close-up photographs has a focal length of 21.5 mm. The farthest it can be placed from the film is 34.0 mm. (a) What is the closest object (in mm) that can be photographed? 58.5 mm (b) What is the magnification of this closest object? 0.581 × ×arrow_forward
- Given two particles with Q = 4.40-µC charges as shown in the figure below and a particle with charge q = 1.40 ✕ 10−18 C at the origin. (Note: Assume a reference level of potential V = 0 at r = ∞.) Three positively charged particles lie along the x-axis of the x y coordinate plane.Charge q is at the origin.Charge Q is at (0.800 m, 0).Another charge Q is at (−0.800 m, 0).(a)What is the net force (in N) exerted by the two 4.40-µC charges on the charge q? (Enter the magnitude.) N(b)What is the electric field (in N/C) at the origin due to the two 4.40-µC particles? (Enter the magnitude.) N/C(c)What is the electrical potential (in kV) at the origin due to the two 4.40-µC particles? kV(d)What If? What would be the change in electric potential energy (in J) of the system if the charge q were moved a distance d = 0.400 m closer to either of the 4.40-µC particles?arrow_forward(a) Where does an object need to be placed relative to a microscope in cm from the objective lens for its 0.500 cm focal length objective to produce a magnification of -25? (Give your answer to at least three decimal places.) 0.42 × cm (b) Where should the 5.00 cm focal length eyepiece be placed in cm behind the objective lens to produce a further fourfold (4.00) magnification? 15 × cmarrow_forwardIn a LASIK vision correction, the power of a patient's eye is increased by 3.10 D. Assuming this produces normal close vision, what was the patient's near point in m before the procedure? (The power for normal close vision is 54.0 D, and the lens-to-retina distance is 2.00 cm.) 0.98 x marrow_forward
- Don't use ai to answer I will report you answerarrow_forwardA shopper standing 2.00 m from a convex security mirror sees his image with a magnification of 0.200. (Explicitly show on paper how you follow the steps in the Problem-Solving Strategy for mirrors found on page 1020. Your instructor may ask you to turn in this work.) (a) Where is his image (in m)? (Use the correct sign.) -0.4 m in front of the mirror ▾ (b) What is the focal length (in m) of the mirror? -0.5 m (c) What is its radius of curvature (in m)? -1.0 marrow_forwardAn amoeba is 0.309 cm away from the 0.304 cm focal length objective lens of a microscope.arrow_forward
- Two resistors of resistances R1 and R2, with R2>R1, are connected to a voltage source with voltage V0. When the resistors are connected in series, the current is Is. When the resistors are connected in parallel, the current Ip from the source is equal to 10Is. Let r be the ratio R1/R2. Find r. I know you have to find the equations for V for both situations and relate them, I'm just struggling to do so. Please explain all steps, thank you.arrow_forwardBheem and Ram, jump off either side of a bridge while holding opposite ends of a rope and swing back and forth under the bridge to save a child while avoiding a fire. Looking at the swing of just Bheem, we can approximate him as a simple pendulum with a period of motion of 5.59 s. How long is the pendulum ? When Bheem swings, he goes a full distance, from side to side, of 10.2 m. What is his maximum velocity? What is his maximum acceleration?arrow_forwardThe position of a 0.300 kg object attached to a spring is described by x=0.271 m ⋅ cos(0.512π⋅rad/s ⋅t) (Assume t is in seconds.) Find the amplitude of the motion. Find the spring constant. Find the position of the object at t = 0.324 s. Find the object's velocity at t = 0.324 s.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College
College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning


Principles of Physics: A Calculus-Based Text
Physics
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers, Technology ...
Physics
ISBN:9781305116399
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

College Physics
Physics
ISBN:9781938168000
Author:Paul Peter Urone, Roger Hinrichs
Publisher:OpenStax College

Physics for Scientists and Engineers: Foundations...
Physics
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Cengage Learning

The Laws of Thermodynamics, Entropy, and Gibbs Free Energy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=8N1BxHgsoOw;License: Standard YouTube License, CC-BY