
Concept explainers
Write chemical equations, showing all necessary reagents, for the preparation of
by each of the following methods:
Hydroboration–oxidation of an
Use of a Grignard reagent
Use of a Grignard reagent in a way different from part (b)
Reduction of a
Hydrogenation of an
Reduction with sodium borohydride
Interpretation:
The chemical equations showing all necessary reagents for the preparation of
Concept introduction:
Hydroboration-oxidation of an alkene results in overall addition of water molecule across the double bond with a regioselectivity opposite to that of Markovnikov’s rule.
In the overall reaction, a hydrogen atom gets attached to the double bonded carbon atom having fewer hydrogens and the hydroxyl group gets attached to the carbon atom having greater number of hydrogens.
When a Grignard reagent reacts with formaldehyde primary alcohols are produced.
Grignard reagents are nucleophilic and react with oxiranes producing alcohols.
Aldehydes are reduced to the corresponding primary alcohols by using
By a suitable reducing agent like lithium aluminum hydride, carboxylic acids are reduced to the corresponding primary alcohols.
Answer to Problem 16P
Solution:
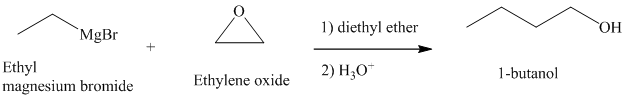

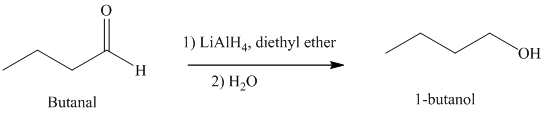
Explanation of Solution
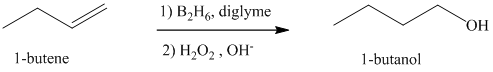
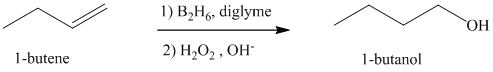
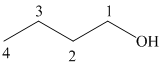
a) Hydroboration-oxidation of an alkene to form
The structure of
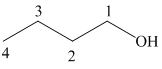
The hydroxyl group is attached to the C1 carbon atom. This alcohol is produced by hydroboration-oxidaton of an alkene. So in the alkene, the C1 carbon atom must be double bonded to the the C2 carbon atom. Thus, the alkene must be
The regioselectivity of hydroboration-oxidation is such that the hydrogen atom will get attached to the C2 carbon atom while the hydroxyl group will get attached to the C1 carbon atom producing
The reaction is shown below:
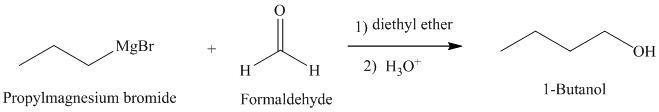
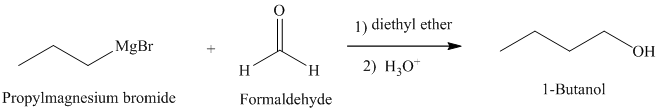
b) Grignard reagent is used to prepare
The structure of
Grignard reagents are nucleophilic and react with carbonyl groups forming a new carbon-carbon bond. An aqueous acid is used to convert the intermediate alkoxy ion to the corresponding alcohol.
In
The reaction is shown below:
c) Different Grignard reagent is used to prepare
The structure of
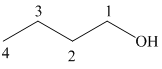
It is a primary alcohol. The hydroxyl group is attached to the C1 carbon atom.
Grignard reagents reacts with ethylene oxide to produce primary alcohol containing two more carbon atoms than the alkyl halide.
Thus, ethyl magnesium bromide, upon reaction with ethylene oxide, will produce
The reaction is shown below:
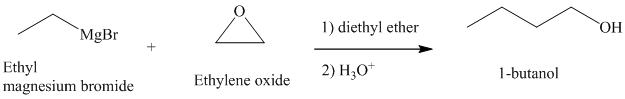
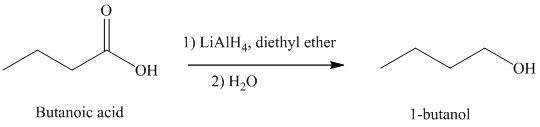
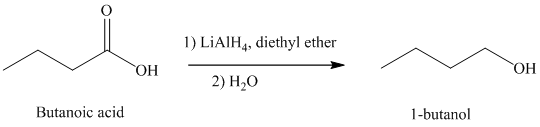
d) The reduction of carboxylic acid to produce

Carboxylic acids, upon reduction, produce the correspionding primary alcohols.
Thus, reduction of butanoic acid with Lithium aluminum hydride will produce
The reaction is shown below:
e) Hydrogenation of an aldehyde to form

Reduction of aldehydes with a suitable reducing agent produces the corresponding primary alcohols.
Thus, reduction of butanal in the presence of Lithium aluminum hydride will produce
The reaction is shown below:
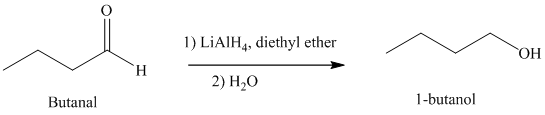
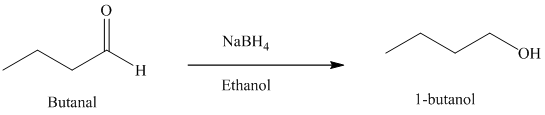
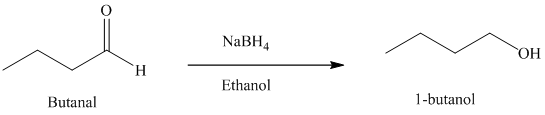
f) Reduction of an aldehyde to form

Sodium borohydride is used for the reduction of aldehydes and ketones to primary and secondary alcohols respectively.
Thus, reduction of butanal with sodium borohydride will produce
The reaction is shown below:
Want to see more full solutions like this?
Chapter 16 Solutions
ORGANIC CHEMISTRY-W/STUD.SOLN.MAN.
- Don't used hand raiting and don't used Ai solutionarrow_forwardDon't used Ai solution and don't used hand raitingarrow_forwardOA. For the structure shown, rank the bond lengths (labeled a, b and c) from shortest to longest. Place your answer in the box. Only the answer in the box will be graded. (2 points) H -CH3 THe b Нarrow_forward
- Don't used hand raitingarrow_forwardQuizzes - Gen Organic & Biological Che... ☆ myd21.lcc.edu + O G screenshot on mac - Google Search savings hulu youtube google disney+ HBO zlib Homework Hel...s | bartleby cell bio book Yuzu Reader: Chemistry G periodic table - Google Search b Home | bartleby 0:33:26 remaining CHEM 120 Chapter 5_Quiz 3 Page 1: 1 > 2 > 3 > 6 ¦ 5 > 4 > 7 ¦ 1 1 10 8 ¦ 9 a ¦ -- Quiz Information silicon-27 A doctor gives a patient 0.01 mC i of beta radiation. How many beta particles would the patient receive in I minute? (1 Ci = 3.7 x 10 10 d/s) Question 5 (1 point) Saved Listen 2.22 x 107 222 x 108 3.7 x 108 2.22 x 108 none of the above Question 6 (1 point) Listen The recommended dosage of 1-131 for a test is 4.2 μCi per kg of body mass. How many millicuries should be given to a 55 kg patient? (1 mCi = 1000 μСi)? 230 mCiarrow_forwardDon't used hand raiting and don't used Ai solutionarrow_forward
- Don't used hand raiting and don't used Ai solutionarrow_forwardQ3: Arrange each group of compounds from fastest SN2 reaction rate to slowest SN2 reaction rate. CI Cl H3C-Cl CI a) A B C D Br Br b) A B C Br H3C-Br Darrow_forwardQ4: Rank the relative nucleophilicity of halide ions in water solution and DMF solution, respectively. F CI Br | Q5: Determine which of the substrates will and will not react with NaSCH3 in an SN2 reaction to have a reasonable yield of product. NH2 Br Br Br .OH Brarrow_forward
- Classify each molecule as optically active or inactive. Determine the configuration at each H соон Chirality center OH 애 He OH H3C Ноос H H COOH A K B.arrow_forwardQ1: Rank the relative nucleophilicity of the following species in ethanol. CH3O¯, CH3OH, CH3COO, CH3COOH, CH3S Q2: Group these solvents into either protic solvents or aprotic solvents. Acetonitrile (CH3CN), H₂O, Acetic acid (CH3COOH), Acetone (CH3COCH3), CH3CH2OH, DMSO (CH3SOCH3), DMF (HCON(CH3)2), CH3OHarrow_forwardDon't used hand raiting and don't used Ai solutionarrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning


