
CHEMISTRY >CUSTOM<
14th Edition
ISBN: 9781259137815
Author: Julia Burdge
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 14.3, Problem 4CP
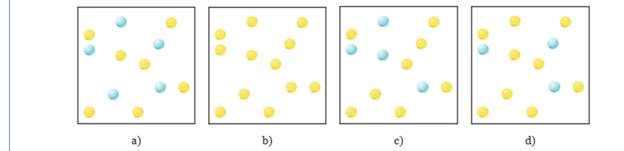
Which figure below represents the numbers of molecules present after 20 s for the reaction in question 14.3.3?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
5. If 44 grams of sodium chloride, NaCI, reacts with an excess amount of
magnesium oxide, MgO, how many grams of sodium oxide, Na,0, will be
produced?
2 Nacl + Mgo → Na,0 + MgCl,
Given:
Find:
Mole ratio:
Mole ratio:
Question 14 of 29>
<
For the chemical reaction
3 KOH H, PO K,PO43 H2O
how many moles of potassium phosphate will be produced from 43.9 g of potassium hydroxide?
moles of potassium phosphate:
mol
help
privacy policy
terms of use
about us
contact us
careers
How do you solve question 12?
Chapter 14 Solutions
CHEMISTRY >CUSTOM<
Ch. 14.1 - Practice Problem ATTEMPT
Write the rate...Ch. 14.1 - Practice ProblemBUILD Write the balanced equation...Ch. 14.1 - Prob. 1PPCCh. 14.1 - 14.1.1 Which expressions are correct for the rate...Ch. 14.1 - 14.1.2 In the same reaction:
if the concentration...Ch. 14.2 - Practice Problem ATTEMPT Consider the reaction:...Ch. 14.2 - Practice Problem BUILD Consider the following...Ch. 14.2 - Practice Problem CONCEPTUALIZE
Consider the...Ch. 14.2 - Answer questions 14.2.1 through 14.2.4 using the...Ch. 14.2 - Answer questions 14.2.1 through 14.2.4 using the...
Ch. 14.2 - Answer questions 14.2.1 through 14.2.4 using the...Ch. 14.2 - Answer questions 14.2.1 through 14.2.4 using the...Ch. 14.2 - 14.2.5 The diagrams represent three experiments in...Ch. 14.3 - Prob. 1PPACh. 14.3 - Practice Problem BUILD
For the following general...Ch. 14.3 - Practice Problem CONCEPTUALIZE
Three initial-rate...Ch. 14.3 - The first-order decomposition of dinitrogen...Ch. 14.3 - The first-order decomposition of dinitrogen...Ch. 14.3 - 14.3.3 Consider the first-order reaction in which...Ch. 14.3 - Which figure below represents the numbers of...Ch. 14.3 - 14.3.5 Of the plots shown here, ___________...Ch. 14.4 - Practice Problem ATTEMPT
The rate constant for the...Ch. 14.4 - Practice Problem BUILD
Refer again to the reaction...Ch. 14.4 - Practice Problem CONCEPTUALIZE
The diagrams on...Ch. 14.4 - Use the table of data collected for a first-order...Ch. 14.4 - Prob. 2CPCh. 14.4 - Prob. 3CPCh. 14.5 - Practice Problem ATTEMPT Ethyl iodide ( C 2 H 5 I)...Ch. 14.5 - Practice Problem BUILD Use the calculated k from...Ch. 14.5 - Practice Problem CONCEPTUALIZE
Use the graph in...Ch. 14.5 - Use the following information to answer questions...Ch. 14.5 - Use the following information to answer questions...Ch. 14.5 - Use the following information to answer questions...Ch. 14.5 - 14.5.4 A plausible mechanism for the reaction:
Ch. 14.6 - Practice ProblemATTEMPT Calculate the half-life of...Ch. 14.6 - Practice ProblemBUILD Calculate the rate constant...Ch. 14.6 - Practice Problem CONCEPTUALIZE
The diagrams show a...Ch. 14.7 - Practice Problem ATTEMPT
The reaction is second...Ch. 14.7 - Practice Problem BUILD
Determine the initial...Ch. 14.7 - Practice ProblemCONCEPTUALIZE The diagrams below...Ch. 14.8 - Practice ProblemATTEMPT The second-order rate...Ch. 14.8 - Practice Problem BUILD Use the graph to determine...Ch. 14.8 - Prob. 1PPCCh. 14.9 - Practice ProblemATTEMPT Use the data in the...Ch. 14.9 - Practice ProblemBUILD Based on the data shown in...Ch. 14.9 - Practice Problem CONCEPTUALIZE
According to the...Ch. 14.10 - Practice ProblemATTEMPT Calculate the rate...Ch. 14.10 - Practice ProblemBUILD Calculate the rate constant...Ch. 14.10 - Practice ProblemCONCEPTUALIZE According to the...Ch. 14.11 - Practice Problem ATTEMPT
The reaction between and...Ch. 14.11 - Practice ProblemBUILD Propose a plausible...Ch. 14.11 - Practice Problem CONCEPTUALIZE
How many steps are...Ch. 14.12 - Practice Problem ATTEMPT
Show that the following...Ch. 14.12 - Practice Problem BUILD
The reaction proceeds via...Ch. 14.12 - Practice Problem CONCEPTUALIZE
The reaction of is...Ch. 14 - Prob. 1KSPCh. 14 - Prob. 2KSPCh. 14 - Prob. 3KSPCh. 14 - Prob. 4KSPCh. 14 - 14.1 What is meant by the rate of a chemical...Ch. 14 - Distinguish between average rate and instantaneous...Ch. 14 - What are the advantages of measuring the initial...Ch. 14 - Identify two reactions that are very slow (take...Ch. 14 - Write the reaction rate expressions for the...Ch. 14 - Write the reaction rate expressions for the...Ch. 14 - Consider the reaction: 2NO ( g ) + O 2 ( g ) → 2NO...Ch. 14 - 14.8 Consider the reaction:
Suppose that at a...Ch. 14 - 14.9 Explain what is meant by the rate law of a...Ch. 14 - Prob. 10QPCh. 14 - What are the units for the rate constants of...Ch. 14 - 14.12 Consider the zeroth-order reaction: a ...Ch. 14 - 14.13 The rate constant of a first-order reaction...Ch. 14 - Identify two reactions that are very slow (take...Ch. 14 - The rate law for the reaction: N H 4 + ( a q )+N O...Ch. 14 - Use the data in Table 14.2 to calculate the rate...Ch. 14 - 14.17 Consider the reaction:
From the following...Ch. 14 - Consider the reaction: X + Y → Z From the...Ch. 14 - Determine the overall orders of the reactions to...Ch. 14 - 14.20 Consider the reaction:
The rate of the...Ch. 14 - Cyclobutane decomposes to ethylene according to...Ch. 14 - The following gas-phase reaction was studied at...Ch. 14 - Write an equation relating the concentration of a...Ch. 14 - 14.24 Define half-life. Write the equation...Ch. 14 - Prob. 25QPCh. 14 - 14.26 For a first-order reaction, how long will it...Ch. 14 - What is the half-life of a compound if 75 percent...Ch. 14 - 14.28 The thermal decomposition of phosphine into...Ch. 14 - The rate constant for the second-order reaction:...Ch. 14 - The rate constant for the second-order reaction:...Ch. 14 - 14.31 The second-order rate constant for the...Ch. 14 - Prob. 32QPCh. 14 - 14.33 The reaction shown here follows first-order...Ch. 14 - 14 34 Define activation energy. What role does...Ch. 14 - Prob. 35QPCh. 14 - Prob. 36QPCh. 14 - The burning of methane in oxygen is a highly...Ch. 14 - Sketch a potential-energy versus reaction progress...Ch. 14 - The reaction H+H 2 → H 2 +H has been studied for...Ch. 14 - Over the range of about ±3°C from normal body...Ch. 14 - For the reaction: NO ( g ) + O 3 ( g ) → NO 2 ( g...Ch. 14 - The rate constant of a first-order reaction is 4...Ch. 14 - The rate constants of some reactions double with...Ch. 14 - 14.44 The rate at which tree crickets chirp is ...Ch. 14 - The rate of bacterial hydrolysis of fish muscle is...Ch. 14 - Prob. 46QPCh. 14 - Given the same reactant concentrations, the...Ch. 14 - 14.48 Variation of the rate constant with...Ch. 14 - 14.49 Diagram A describes the initial state of...Ch. 14 - 14 50 What do we mean by the mechanism of a...Ch. 14 - 14.51 What is an elementary step? What is the...Ch. 14 - 14.52 Classify the following elementary reactions...Ch. 14 - Reactions can be classified as unimolecular,...Ch. 14 - Determine the molecularity, and write the rate law...Ch. 14 - 14.55 What is the rate-determining step of a...Ch. 14 - 14.56 The equation for the combustion of ethane ...Ch. 14 - Specify which of the following species cannot be...Ch. 14 - Classify each of the following elementary steps as...Ch. 14 - 14.59 The rate law for the reaction:
is given by...Ch. 14 - For the reaction x 2 + y + z → x y + x z , it is...Ch. 14 - The rate law for the reaction: 2H 2 ( g ) + 2NO (...Ch. 14 - 14.62 The rate law for the decomposition of ozone...Ch. 14 - 14.63 How does a catalyst increase the rate of a...Ch. 14 - 14.64 What are the characteristics of a...Ch. 14 - A certain reaction is known to proceed slowly at...Ch. 14 - Most reactions, including enzyme-catalyzed...Ch. 14 - 14.67 Are enzyme-catalyzed reactions examples of...Ch. 14 - The concentrations of enzymes in cells are usually...Ch. 14 - When fruits such as apples and pears are cut. the...Ch. 14 - The first-order rate constant for the dehydration...Ch. 14 - Which two potential-energy profiles represent the...Ch. 14 - Consider the following mechanism for the...Ch. 14 - List four factors that influence the rate of a...Ch. 14 - 14.71 Suggest experimental means by which the...Ch. 14 - 14.75 “The rate constant for the reaction:
is .”...Ch. 14 - Prob. 76APCh. 14 - The following diagrams represent the progress of...Ch. 14 - The following diagrams show the progress of the...Ch. 14 - Prob. 79APCh. 14 - Prob. 80APCh. 14 - 14.81 When methyl phosphate is heated in acid...Ch. 14 - The rate of the reaction: CH 3 COOC 2 H 5 ( a q )...Ch. 14 - Explain why most metals used in catalysis are...Ch. 14 - Prob. 84APCh. 14 - The bromination of acetone is acid-catalyzed: CH 3...Ch. 14 - The decomposition of N 2 O to N 2 and O 2 is a...Ch. 14 - 14.87 The reaction proceeds slowly in aqueous...Ch. 14 - Prob. 88APCh. 14 - The integrated rate law for the zeroth-order...Ch. 14 - 14.90 A flask contains a mixture of compounds A...Ch. 14 - Prob. 91APCh. 14 - 14.92 The rate law for the reaction . Which of the...Ch. 14 - 14.93 The reaction of to form 2EG is exothermic,...Ch. 14 - 14.94 The activation energy for the decomposition...Ch. 14 - Prob. 95APCh. 14 - 14.96 When 6 g of granulated Zn is added to a...Ch. 14 - Prob. 97APCh. 14 - 14.98 A certain first-order reaction is 35.5...Ch. 14 - 14.99 The decomposition of dinitrogen pentoxide...Ch. 14 - 14.100 The thermal decomposition of obeys...Ch. 14 - 14.101 When a mixture of methane and bromine is...Ch. 14 - 14.102 The rate of the reaction between to form...Ch. 14 - The rate constant for the gaseous reaction: H 2 (...Ch. 14 - A gas mixture containing CH 3 fragments. C 2 H 6...Ch. 14 - Consider the following elementary step: X + 2Y →...Ch. 14 - 14.106 The following scheme in which A is...Ch. 14 - 14.107 (a) Consider two reactions, A and B. If the...Ch. 14 - The rate law for the following reaction: CO ( g )...Ch. 14 - Consider the following elementary steps for a...Ch. 14 - Prob. 110APCh. 14 - Consider the following potential-energy profile...Ch. 14 - The rate of a reaction was followed by the...Ch. 14 - 14.113 The first-order rate constant for the...Ch. 14 - 14.114 Many reactions involving heterogeneous...Ch. 14 - Thallium(I) is oxidized by cerium(IV) as follows:...Ch. 14 - The activation energy for the reaction: N 2 O ( g...Ch. 14 - Δ H ° for the reaction in Problem 14.116 is -164...Ch. 14 - 14.118 At a certain elevated temperature, ammonia...Ch. 14 - 14.119 The following expression shows the...Ch. 14 - In a certain industrial process involving a...Ch. 14 - Strontium-90, a radioactive isotope, is a major...Ch. 14 - Prob. 122APCh. 14 - Prob. 123APCh. 14 - A factory that specializes in the refinement of...Ch. 14 - 14.125 When the concentration of A in the reaction...Ch. 14 - 14.126 The activity of a radioactive sample is the...Ch. 14 - Prob. 127APCh. 14 - Prob. 128APCh. 14 - Prob. 129APCh. 14 - Prob. 130APCh. 14 - Prob. 131APCh. 14 - Prob. 132APCh. 14 - Prob. 133APCh. 14 - 14.134 At a certain elevated temperature, ammonia...Ch. 14 - Polyethylene is used in many items, including...Ch. 14 - In recent years, ozone in the stratosphere has...Ch. 14 - Metastron, an aqueous solution of 89 SrCl 2 , is a...Ch. 14 - Metastron, an aqueous solution of 89 SrCl 2 , is a...Ch. 14 - Metastron, an aqueous solution of 89 SrCl 2 , is a...Ch. 14 - Metastron, an aqueous solution of 89 SrCl 2 , is a...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Carbon disulfide burns in air, producing carbon dioxide and sulfur dioxide. CS2(l)+3O2(g)CO2(g)+2SO2(g);H=1077kJ What is H for the following equation? 12CO2(g)+SO2(g)12CS2(l)+32O2(g)arrow_forwardA lead ore, galena, consisting mainly of lead(II) sulfide, is the principal source of lead. To obtain the lead, the ore is first heated in the air to form lead oxide. PbS(s)+32 O2(g)PbO(s)+SO2(g)H=415.4kJ The oxide is then reduced to metal with carbon. PbO(s)+C(s)Pb(s)+CO(g)H=+108.5kJCalculate H for the reaction of one mole of lead(II) sulfide with oxygen and carbon, forming lead, sulfur dioxide, and carbon monoxide.arrow_forwardHow many moles of W are needed to react with 1.0 mol of Ho in the hypothetic chemical reaction? 3W + 2Ho → 2W3Ho a. 0 b. 1.5 c. 2 d. 3 e. 1arrow_forward
- Adipic acid, a raw material for nylon, is made industrially by the oxidation of cyclohexane. 5 O2 + 2 C6H12 ----------------------> 2 C6H10O4 + 2 H2O cyclohexane special conditions adipic acid How many moles of oxygen would be needed to make 40.0 moles of adipic acid by this reaction? If 164 grams of cyclohexane is used, what is the theoretical yield of adipic acid in moles? In grams? If a given mixture was supposed to produce 74 grams of adipic acid but you only obtained 60 grams, what is the yield of the reaction?arrow_forwardComplete the ICF (BCA) table below for the hypothetical chemical reaction. All values are in moles. Enter all values with three digits (like shown) and include negative or positive signs for the "Change" row. A + 2B ЗС + D A C INITIAL 5.00 2.00 0.00 0.00 CHANGEarrow_forwardRead the given chemical reaction.C2H6 + O2 → CO2 + H2OHow many moles of O2 are required to react completely with 3.2 moles of C2H6? 3.5 moles of O2 6.5 moles of O2 10.4 moles of O2 11.2 moles of O2arrow_forward
- 12:54 PM Sun Oct 22 Question 21.d of 34 The following reaction can be used to convert carbon dioxide to oxygen gas. 4 KO₂ (s) + 2 CO₂(g) → 2 K₂CO3(s) + 3 O₂(g) The theoretical yield for the reaction is 0.232 g O₂. Given that the reaction has a percent yield of 83.4%, what is the mass in g of oxygen gas that is actually produced? Tap here or pull up for additional resourcesarrow_forwardQuestion Completion Status: A Moving to another question will save this response. Question 7 Consider the following reaction: 2H2O2(aq) → 2H2O(l) +O2(g) When 1.0 g of Kl is added to the H202, bubbles of O2 are produced at an increased rate. When the reaction is complete, the mass of KI is 1.0 g. The KI is a A Moving to another question will save this response.arrow_forwardThe actual yield can only be determined by: 8°F Mostly cloudy O Writing a balanced equation. O Calculating the theoretical yield and plugging into the percent yield. O Reviewing past reactions. O Carrying out the reaction in a laboratory. O Search Harrow_forward
- CHAPTER 4-STOICHIOMETRY: QUANTITATIVE INFORMATION ABOUT CHEMIC Previous Page 3 of 4 Next → Iron ore is converted to iron metal in a reaction with carbon. If 4.00 mol of Fe2O3 is used, what amounts of Fe and CO2 are produced? Fill in the amounts table with your responses. Q Equation Initial (mol) Change (mol) Final (mol) 2 2 Fe₂O3(s) + 3 C(s) 11 W S 4.00 -4.00 0 tv 6.00 - 6.00 o E 0 S + 18 3 4 W AS R 4 Fe(s) Document3.docx. 0 % 6.5⁰ PACUPSTA PXnerienc IL.OMBI wenarchive T + MacBook Pro 6 Y & ★ 7 3 CO2 (9) 0 Yesterday at 6:04 AM AUO 75 70177 21 10.54 AM ★ U C Online t.... * 00 9arrow_forwardGiven the following reaction: N2(g) + O2 (g) ⟶⟶ 43.2 kcal + 2NO(g) If 15.0g NO are produced, how many kcal were absorbed? A. 10.8 B. 15.0 C. 21.6 D. 115 E. 12.3arrow_forwardHelp Fast Pleaseeeeearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning  Chemistry for Engineering StudentsChemistryISBN:9781285199023Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781285199023Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:9781285199023
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning
Calorimetry Concept, Examples and Thermochemistry | How to Pass Chemistry; Author: Melissa Maribel;https://www.youtube.com/watch?v=nSh29lUGj00;License: Standard YouTube License, CC-BY