
Concept explainers
(a)
Interpretation:
The IUPAC name for the products has to be written.
Concept Introduction:
Hydrolysis of Ester:
The reaction in which a bond is broken by the addition of water molecule is called as hydrolysis.
Hydrolysis of esters yields the
The general reaction of ester hydrolysis is shown below,
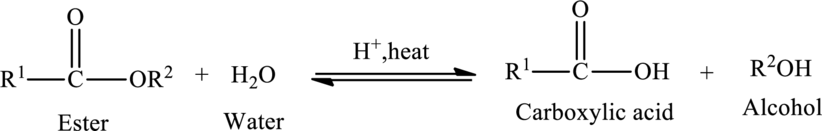
In case of base-catalyzed hydrolysis of an ester, the carboxylic acid does not exist. The reaction results in the formation of salt of carboxylic acid containing the cation of the base catalyst.
(b)
Interpretation:
The given reaction has to be completed by drawing its product structure.
Concept Introduction:
Hydrolysis of Ester:
The reaction in which a bond is broken by the addition of water molecule is called as hydrolysis.
Hydrolysis of esters yields the carboxylic acid and an alcohol. The heat is required to initiate the reaction and a small amount of acid
The general reaction of ester hydrolysis is shown below,
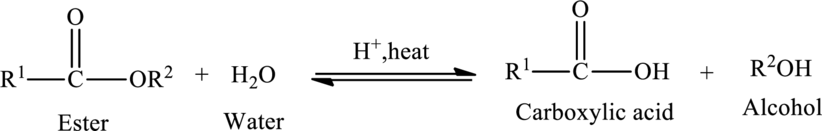
In case of base-catalyzed hydrolysis of an ester, the carboxylic acid does not exist. The reaction results in the formation of salt of carboxylic acid containing the cation of the base catalyst.
(c)
Interpretation:
The given reaction has to be completed by drawing its product structure.
Concept Introduction:
Hydrolysis of Ester:
The reaction in which a bond is broken by the addition of water molecule is called as hydrolysis.
Hydrolysis of esters yields the carboxylic acid and an alcohol. The heat is required to initiate the reaction and a small amount of acid
The general reaction of ester hydrolysis is shown below,
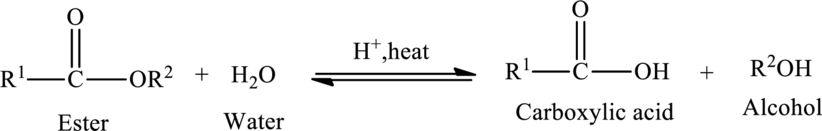
In case of base-catalyzed hydrolysis of an ester, the carboxylic acid does not exist. The reaction results in the formation of salt of carboxylic acid containing the cation of the base catalyst.
(d)
Interpretation:
The given reaction has to be completed by drawing its product structure.
Concept Introduction:
Hydrolysis of Ester:
The reaction in which a bond is broken by the addition of water molecule is called as hydrolysis.
Hydrolysis of esters yields the carboxylic acid and an alcohol. The heat is required to initiate the reaction and a small amount of acid
The general reaction of ester hydrolysis is shown below,
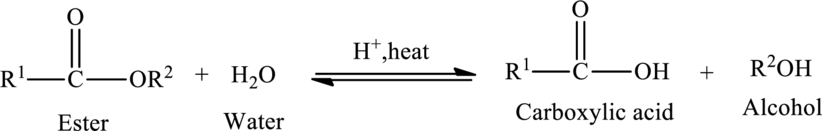
In case of base-catalyzed hydrolysis of an ester, the carboxylic acid does not exist. The reaction results in the formation of salt of carboxylic acid containing the cation of the base catalyst.
Want to see the full answer?
Check out a sample textbook solution
Chapter 14 Solutions
GENERAL, ORGANIC,BIO CHP.10-23-ACCESS>I
- The combustion of 28.8 g of NH3 consumes exactly _____ g of O2. 4 NH3 + 7 O2 ----> 4 NO2 + 6 H2Oarrow_forwardWhat is the molecular formula of the bond-line structure shown below OH HO ○ C14H12O2 ○ C16H14O2 ○ C16H12O2 O C14H14O2arrow_forwardCheck all molecules that are acids on the list below. H2CO3 HC2H3O2 C6H5NH2 HNO3 NH3arrow_forward
- From the given compound, choose the proton that best fits each given description. a CH2 CH 2 Cl b с CH2 F Most shielded: (Choose one) Least shielded: (Choose one) Highest chemical shift: (Choose one) Lowest chemical shift: (Choose one) ×arrow_forwardConsider this molecule: How many H atoms are in this molecule? How many different signals could be found in its 1H NMR spectrum? Note: A multiplet is considered one signal.arrow_forwardFor each of the given mass spectrum data, identify whether the compound contains chlorine, bromine, or neither. Compound m/z of M* peak m/z of M + 2 peak ratio of M+ : M + 2 peak Which element is present? A 122 no M + 2 peak not applicable (Choose one) B 78 80 3:1 (Choose one) C 227 229 1:1 (Choose one)arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





