
Concept explainers
NMR spectra for four isomeric alkyl bromides with the formula
Assign structures to each of the alkyl bromides and assign the peaks in each spectrum.
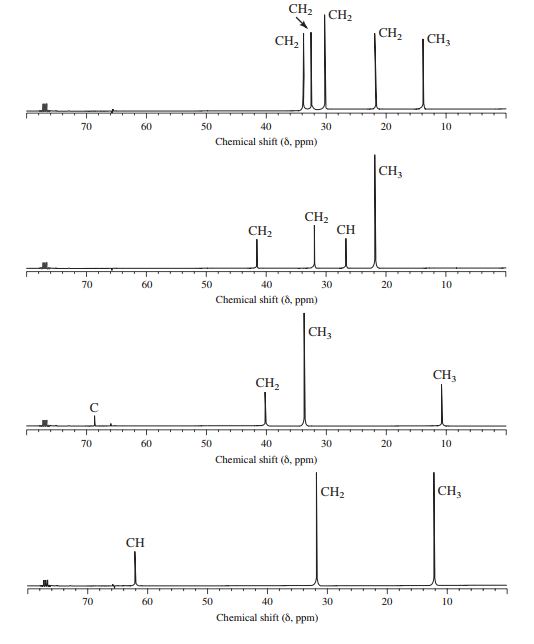
Interpretation:
Structures of each of the isomeric alkyl bromides with the formula
Concept introduction:
In
Carbon atoms having equivalent environment will have the same chemical shift.
In simple molecules, equivalent carbon atoms can be identified just by inspection.
The number of signals depends on the number of distinct carbon atoms in the structure of the compound.
If the structure is symmetrical, the number of signals in the
The electronegative atom/group decreases the shielding of the carbon atom to which it is attached.
In
An
The chemical shift for methyl carbon atoms ranges from
The chemical shift for methylene carbon atoms ranges from
The chemical shift for methine carbon atoms ranges from
The chemical shift for aromatic carbon atoms ranges from
The index of hydrogen deficiency is calculated as follows:
Here
Answer to Problem 43P
Solution:
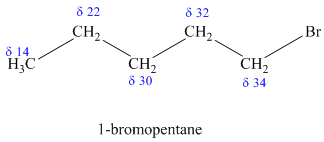
a)
b)
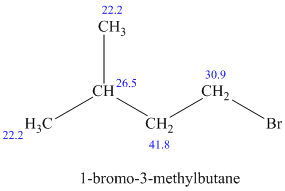
c)
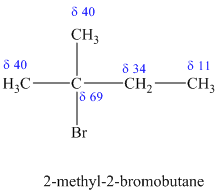
d)
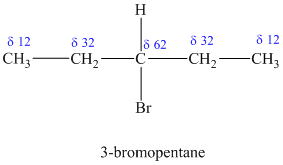
Explanation of Solution
a)
The molecular formula shows an index of hydrogen deficiency equal to zero. Thus, the compound contains ring or multiple bonds. All the carbon atoms must be
The signal at
Combining all this, the only possible structure for the isomer having the formula

b)
The molecular formula shows an index of hydrogen deficiency equal to zero. Thus, the compound contains ring or multiple bonds. All the carbon atoms must be
The signal at
The signal at
Combining all this, the only possible structure for the isomer having the formula
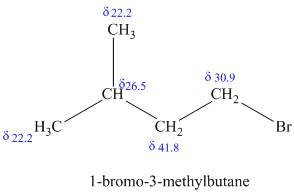
c)
The molecular formula shows an index of hydrogen deficiency equal to zero. Thus, the compound contains ring or multiple bonds. All the carbon atoms must be
The signal at
The signal at
The chemical shift values for the remaining two signals indicate two types of methyl groups. The signal at
Combining all this, the only possible structure for the isomer having the formula

d)
The molecular formula shows an index of hydrogen deficiency equal to zero. Thus, the compound contains ring or multiple bonds. All the carbon atoms must be
The signal at
The signal at
Combining all this, the only possible structure for the isomer having the formula

Want to see more full solutions like this?
Chapter 14 Solutions
CAREY: ORGANIC CHEMISTRY
- Using line angle formulas, draw thestructures of and name four alkanes that have total of 7carbons, one of which is tertiary.Please explain this in detail and can you also explain how to approach a similar problem like this as well?arrow_forwardUsing dashed line wedge projections drawthe indicated compounds and indicate whether thecompound you have drawn is R or S.(a) The two enantiomers of 2-chlorobutane. Can you please explain your steps and how you would approach a similar problem. Thank you!arrow_forward5) There are no lone pairs shown in the structure below. Please add in all lone pairs and then give the hybridization scheme for the compound. (8) 10,11 7) 1.2.3 H 4 | 14 8) COC 12 13 H 16 15 H7 9) - 5.6 C 8 H 10) H 1). 2) 3)_ 11) 12) 13) 4)_ 14) 5) 15) 16) 6)arrow_forward
- The sum of the numbers in the name of isA. 11; B. 13; C. 10; D. 12; E. none of the other answers iscorrect. I believe the awnser should be E to this problem but the solution to this problem is D 12. I'm honestly unsure how that's the solution. If you can please explain the steps to this type of problem and how to approach a problem like this it would be greatly appreciated!arrow_forwardConsider the following data for phosphorus: g atomic mass 30.974 mol electronegativity 2.19 kJ electron affinity 72. mol kJ ionization energy 1011.8 mol kJ heat of fusion 0.64 mol You may find additional useful data in the ALEKS Data tab. Does the following reaction absorb or release energy? 2+ + (1) P (g) + e → P (g) Is it possible to calculate the amount of energy absorbed or released by reaction (1) using only the data above? If you answered yes to the previous question, enter the amount of energy absorbed or released by reaction (1): Does the following reaction absorb or release energy? 00 release absorb Can't be decided with the data given. yes no ☐ kJ/mol (²) P* (8) + + + e →>> P (g) Is it possible to calculate the amount of energy absorbed or released by reaction (2) using only the data above? If you answered yes to the previous question, enter the amount of energy absorbed or released by reaction (2): ☐ release absorb Can't be decided with the data given. yes no kJ/mol аarrow_forwardThe number of hydrogens in an alkyne that has a main chain of 14carbons to which are attached a cyclobutyl ring, a benzene ring, an–OH group, and a Br is A. 34; B. 35; C. 36; D. 24; E. 43arrow_forward
- Hello! I have a 500 Hz H-NMR for 1,5-bis-(4-methoxyphenyl)-penta-1,4-dien-3-one. I need to label the signals with the corresponding H's. Then, find out if the two alkenes are cis or trans by calculating the J values. I believe that I have the H-NMR labeled correctly, but not sure if I got the J values correct to determine if the two alkenes in the compound will make the compound cis or trans.arrow_forwardWhat is the only possible H-Sb-H bond angle in SbH3?arrow_forwardpls helparrow_forward

 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole



