
Concept explainers
Each of the following compounds is characterized by a 1H NMR spectrum that consists of
only a single peak having the chemical shift indicated. Identify each compound.
Interpretation:
The compounds gives only single peak in 1H NMR spectrum as indicated by the chemical shift is to be identified.
Concept introduction:
The information related to the proton in the compound can be deduced by the number of signals in a spectrum.
The proton chemical shift in the compound is due to the environment of the proton.
The signal of a particular proton can be split by the presence of protons in vicinal position.
The NMR spectrum of a compound will consist of a single peak if all protons in the compound are structurally equivalent.
Index of hydrogen deficiency is calculated as
Here
Oxygen atoms do not affect the index of hydrogen deficiency.
Answer to Problem 31P
Solution:
Explanation of Solution
Chemical formula:
A single peak at
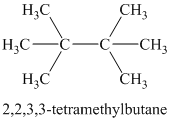
Therefore, the structure of the compound is:
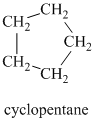
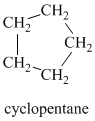
Chemical formula:
A single peak at
Therefore, it must be a symmetric cycloalkane:
Chemical formula:
A single peak at
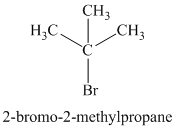
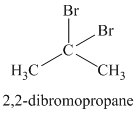
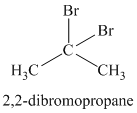
Chemical formula:
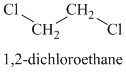
The chemical formula shows it is a saturated alkyl halide. A single peak means that all protons are equivalent. The presence of the bromine atoms accounts for the higher chemical shift of the protons. Therefore, the structure of the compound is:
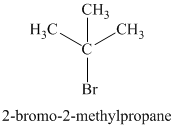
Chemical formula:
A single peak shows all protons to be equivalent. The formula is of a saturated alkyl halide. The presence of two chlorine atoms on the same carbon as the protons will increase the shift to
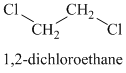
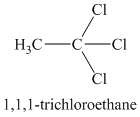
Chemical formula:
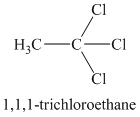
A single peak means all protons must be equivalent. The chemical formula shows it to be a saturated alkyl halide. The higher shift of
Chemical formula:
A single peak shows all protons to be equivalent. The chemical formula shows it to be a saturated alkyl halide. The high chemical shift of
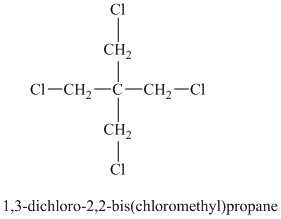
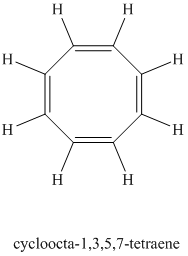
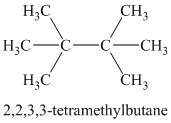
Chemical formula:
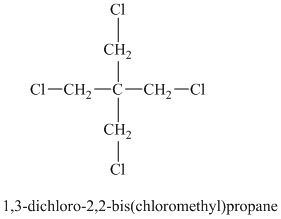
A single peak shows all protons are equivalent. The chemical formula shows an index of hydrogen deficiency of four. This, taken together with a chemical shift of
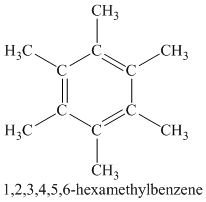
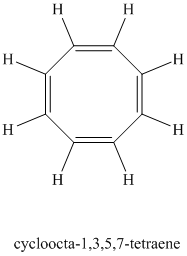
Chemical formula:
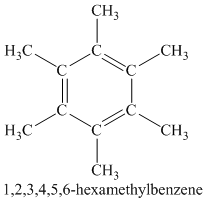
A single peak shows all protons are equivalent. The higher chemical shift of
Therefore, the structure of the compound is:
Want to see more full solutions like this?
Chapter 14 Solutions
CAREY: ORGANIC CHEMISTRY
- Problem 5-31 Which of the following objects are chiral? (a) A basketball (d) A golf club (b) A fork (c) A wine glass (e) A spiral staircase (f) A snowflake Problem 5-32 Which of the following compounds are chiral? Draw them, and label the chirality centers. (a) 2,4-Dimethylheptane (b) 5-Ethyl-3,3-dimethylheptane (c) cis-1,4-Dichlorocyclohexane Problem 5-33 Draw chiral molecules that meet the following descriptions: (a) A chloroalkane, C5H11Cl (c) An alkene, C6H12 (b) An alcohol, C6H140 (d) An alkane, C8H18 Problem 5-36 Erythronolide B is the biological precursor of erythromycin, a broad-spectrum antibiotic. How H3C CH3 many chirality centers does erythronolide B have? OH Identify them. H3C -CH3 OH Erythronolide B H3C. H3C. OH OH CH3arrow_forwardPLEASE HELP! URGENT! PLEASE RESPOND!arrow_forward2. Propose a mechanism for this reaction. ہلی سے ملی N H (excess)arrow_forward
- Steps and explanationn please.arrow_forwardProblem 5-48 Assign R or S configurations to the chirality centers in ascorbic acid (vitamin C). OH H OH HO CH2OH Ascorbic acid O H Problem 5-49 Assign R or S stereochemistry to the chirality centers in the following Newman projections: H Cl H CH3 H3C. OH H3C (a) H H H3C (b) CH3 H Problem 5-52 Draw the meso form of each of the following molecules, and indicate the plane of symmetry in each: OH OH (a) CH3CHCH2CH2CHCH3 CH3 H3C. -OH (c) H3C CH3 (b) Problem 5-66 Assign R or S configurations to the chiral centers in cephalexin, trade-named Keflex, the most widely prescribed antibiotic in the United States. H2N H IHH S Cephalexin N. CH3 CO₂Harrow_forwardSteps and explanationn please.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
