
Bundle: College Physics, Loose-Leaf Version, 11th + WebAssign Printed Access Card for Serway/Vuille's College Physics, 11th Edition, Multi-Term
11th Edition
ISBN: 9781337741620
Author: Raymond A. Serway, Chris Vuille
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Textbook Question
thumb_up100%
Chapter 12.3, Problem 12.2QQ
Identify the paths A, B, C, and D in Figure 12.11 as isobaric, isothermal, isovolumetric, or adiabatic. For path B, Q = 0.
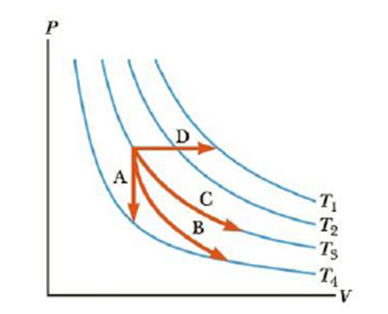
Figure 12.11 (Quick Quiz 12.2) Identify the nature of paths A, B, C, and D
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Please don't use Chatgpt will upvote and give handwritten solution
how would i express force in vector form I keep getting a single number
please help me solve this questions. show all calculations and a good graph too :)
Chapter 12 Solutions
Bundle: College Physics, Loose-Leaf Version, 11th + WebAssign Printed Access Card for Serway/Vuille's College Physics, 11th Edition, Multi-Term
Ch. 12.1 - By visual inspection, order the PV diagrams shown...Ch. 12.3 - Identify the paths A, B, C, and D in Figure 12.11...Ch. 12.4 - Three engines operate between reservoirs separated...Ch. 12.5 - Which of the following is true for the entropy...Ch. 12.5 - Prob. 12.5QQCh. 12 - Two identical containers each hold 1 mole of an...Ch. 12 - Which one of the following statements is true? (a)...Ch. 12 - Prob. 3CQCh. 12 - Prob. 4CQCh. 12 - For an ideal gas in an isothermal process, there...
Ch. 12 - An ideal gas undergoes an adiabatic process so...Ch. 12 - Is it possible to construct a heat engine that...Ch. 12 - A heat engine does work Weng while absorbing...Ch. 12 - When a sealed Thermos bottle full of hot coffee is...Ch. 12 - The first law of thermodynamics is U = Q + W. For...Ch. 12 - The first law of thermodynamics says we cant get...Ch. 12 - Objects A and B with TA TB are placed in thermal...Ch. 12 - Prob. 13CQCh. 12 - Prob. 14CQCh. 12 - An ideal gas is compressed to half its initial...Ch. 12 - A thermodynamic process occurs in which the...Ch. 12 - Prob. 17CQCh. 12 - An ideal gas is enclosed in a cylinder with a...Ch. 12 - Sketch a PV diagram and find the work done by the...Ch. 12 - Gas in a container is at a pressure of 1.5 atm and...Ch. 12 - Find the numeric value of the work done on the gas...Ch. 12 - A gas expands from I to F along the three paths...Ch. 12 - A gas follows the PV diagram in Figure P12.6. Find...Ch. 12 - A sample of helium behaves as an ideal gas as it...Ch. 12 - (a) Find the work done by an ideal gas as it...Ch. 12 - One mole of an ideal gas initially at a...Ch. 12 - (a) Determine the work done on a fluid that...Ch. 12 - A balloon holding 5.00 moles of helium gas absorbs...Ch. 12 - A chemical reaction transfers 1250 J of thermal...Ch. 12 - Prob. 13PCh. 12 - A cylinder of volume 0.300 m3 contains 10.0 mol of...Ch. 12 - A gas expands from I to F in Figure P12.5. The...Ch. 12 - In a running event, a sprinter does 4.8 105 J of...Ch. 12 - A gas is compressed at a constant pressure of...Ch. 12 - A quantity of a monatomic ideal gas undergoes a...Ch. 12 - A gas is enclosed in a container fitted with a...Ch. 12 - A monatomic ideal gas under-goes the thermodynamic...Ch. 12 - An ideal gas is compressed from a volume of Vi =...Ch. 12 - A system consisting of 0.025 6 moles of a diatomic...Ch. 12 - An ideal monatomic gas expands isothermally from...Ch. 12 - An ideal gas expands at constant pressure. (a)...Ch. 12 - An ideal monatomic gas contracts in an isobaric...Ch. 12 - An ideal diatomic gas expands adiabatically from...Ch. 12 - An ideal monatomic gas is contained in a vessel of...Ch. 12 - Consider the cyclic process described by Figure...Ch. 12 - A 5.0-kg block of aluminum is heated from 20C to...Ch. 12 - One mole of gas initially at a pressure of 2.00...Ch. 12 - A gas increases in pressure from 2.00 atm to 6.00...Ch. 12 - An ideal gas expands at a constant pressure of...Ch. 12 - A heat engine operates between a reservoir at 25C...Ch. 12 - A heat engine is being designed to have a Carnot...Ch. 12 - The work done by an engine equals one-fourth the...Ch. 12 - In each cycle of its operation, a heat engine...Ch. 12 - One of the most efficient engines ever built is a...Ch. 12 - A lawnmower engine ejects 1.00 104 J each second...Ch. 12 - An engine absorbs 1.70 kJ from a hot reservoir at...Ch. 12 - A heat pump has a coefficient of performance of...Ch. 12 - A freezer has a coefficient of performance of...Ch. 12 - Prob. 42PCh. 12 - In one cycle a heat engine absorbs 500 J from a...Ch. 12 - A power plant has been proposed that would make...Ch. 12 - Prob. 45PCh. 12 - A heat engine operates in a Carnot cycle between...Ch. 12 - A Styrofoam cup holding 125 g of hot water at 1.00...Ch. 12 - A 65-g ice cube is initially at 0.0C. (a) Find the...Ch. 12 - A freezer is used to freeze 1.0 L of water...Ch. 12 - What is the change in entropy of 1.00 kg of liquid...Ch. 12 - A 70.0-kg log falls from a height of 25.0 m into a...Ch. 12 - A sealed container holding 0.500 kg of liquid...Ch. 12 - Prob. 53PCh. 12 - When an aluminum bar is temporarily connected...Ch. 12 - Prepare a table like Table 12.3 for the following...Ch. 12 - Prob. 56PCh. 12 - Prob. 57PCh. 12 - Prob. 58PCh. 12 - Sweating is one of the main mechanisms with which...Ch. 12 - Prob. 60PCh. 12 - Suppose a highly trained athlete consumes oxygen...Ch. 12 - A Carnot engine operates between the temperatures...Ch. 12 - Prob. 63APCh. 12 - A Carnot engine operates between 100C and 20C. How...Ch. 12 - A substance undergoes the cyclic process shown in...Ch. 12 - When a gas follows path 123 on the PV diagram in...Ch. 12 - Prob. 67APCh. 12 - An ideal gas initially at pressure P0, volume V0,...Ch. 12 - One mole of neon gas is heated from 300. K to 420....Ch. 12 - Every second at Niagara Falls, approximately 5.00 ...Ch. 12 - A cylinder containing 10.0 moles of a monatomic...Ch. 12 - Prob. 72APCh. 12 - Suppose you spend 30.0 minutes on a stair-climbing...Ch. 12 - Hydrothermal vents deep on the ocean floor spout...Ch. 12 - An electrical power plant has an overall...Ch. 12 - A diatomic ideal gas expands from a volume of VA =...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- What is the force (in N) on the 2.0 μC charge placed at the center of the square shown below? (Express your answer in vector form.) 5.0 με 4.0 με 2.0 με + 1.0 m 1.0 m -40 με 2.0 μCarrow_forwardWhat is the force (in N) on the 5.4 µC charge shown below? (Express your answer in vector form.) −3.1 µC5.4 µC9.2 µC6.4 µCarrow_forwardAn ideal gas in a sealed container starts out at a pressure of 8900 N/m2 and a volume of 5.7 m3. If the gas expands to a volume of 6.3 m3 while the pressure is held constant (still at 8900 N/m2), how much work is done by the gas? Give your answer as the number of Joules.arrow_forward
- The outside temperature is 25 °C. A heat engine operates in the environment (Tc = 25 °C) at 50% efficiency. How hot does it need to get the high temperature up to in Celsius?arrow_forwardGas is compressed in a cylinder creating 31 Joules of work on the gas during the isothermal process. How much heat flows from the gas into the cylinder in Joules?arrow_forwardThe heat engine gives 1100 Joules of energy of high temperature from the burning gasoline by exhausting 750 Joules to low-temperature . What is the efficiency of this heat engine in a percentage?arrow_forward
- L₁ D₁ L₂ D2 Aluminum has a resistivity of p = 2.65 × 10 8 2. m. An aluminum wire is L = 2.00 m long and has a circular cross section that is not constant. The diameter of the wire is D₁ = 0.17 mm for a length of L₁ = 0.500 m and a diameter of D2 = 0.24 mm for the rest of the length. a) What is the resistance of this wire? R = Hint A potential difference of AV = 1.40 V is applied across the wire. b) What is the magnitude of the current density in the thin part of the wire? Hint J1 = c) What is the magnitude of the current density in the thick part of the wire? J₂ = d) What is the magnitude of the electric field in the thin part of the wire? E1 = Hint e) What is the magnitude of the electric field in the thick part of the wire? E2 =arrow_forwardplease helparrow_forwardA cheetah spots a gazelle in the distance and begins to sprint from rest, accelerating uniformly at a rate of 8.00 m/s^2 for 5 seconds. After 5 seconds, the cheetah sees that the gazelle has escaped to safety, so it begins to decelerate uniformly at 6.00 m/s^2 until it comes to a stop.arrow_forward
- A projectile is fired with an initial speed of 40.2 m/s at an angle of 35.0 degree above the horizontal on a long flat firing range. Determine. please help and show work for them so i can understand.arrow_forwardpls helparrow_forwardJ K L The graph in the figure shows the position of an object as a function of time. The letters H-L represent particular moments of time. At which moments shown (H, I, etc.) is the speed of the object the greatest? + Position H I K Timearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College
College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

Physics for Scientists and Engineers: Foundations...
Physics
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Cengage Learning

College Physics
Physics
ISBN:9781938168000
Author:Paul Peter Urone, Roger Hinrichs
Publisher:OpenStax College

Principles of Physics: A Calculus-Based Text
Physics
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers, Technology ...
Physics
ISBN:9781305116399
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers with Modern ...
Physics
ISBN:9781337553292
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning
The Second Law of Thermodynamics: Heat Flow, Entropy, and Microstates; Author: Professor Dave Explains;https://www.youtube.com/watch?v=MrwW4w2nAMc;License: Standard YouTube License, CC-BY