
Concept explainers
Write structural formulas and give the IUPAC names for all the isomers of
Interpretation:
The structural formulas and IUPAC names for all the isomers of
Concept introduction:
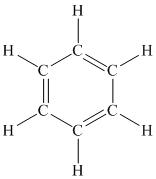
A benzene ring is a cyclic six membered ring containing single and double bonds alternately between carbon atoms. One hydrogen atom is attached to each carbon atom.
In a mono substituted benzene ring, one hydrogen atom is replaced by a substituent.
The substituent should be such that each carbon atom must have formed four bonds and other atoms, if any, should have complete octets.
Answer to Problem 33P
Solution:
Explanation of Solution
The structure of a benzene ring is as follows:
One hydrogen atom is replaced by the group
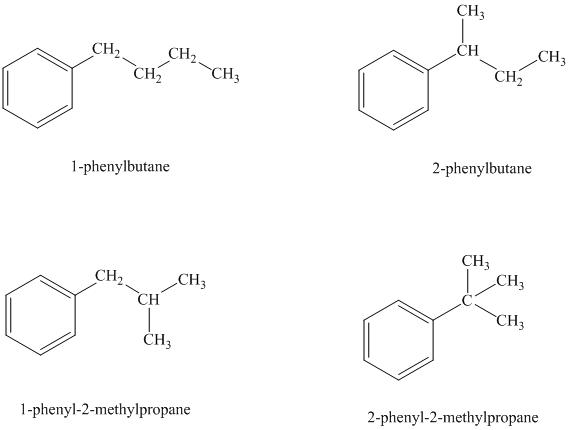
There are four possible ways in which this group can be attached to the ring.
The structures and IUPAC names of the isomers are shown below:
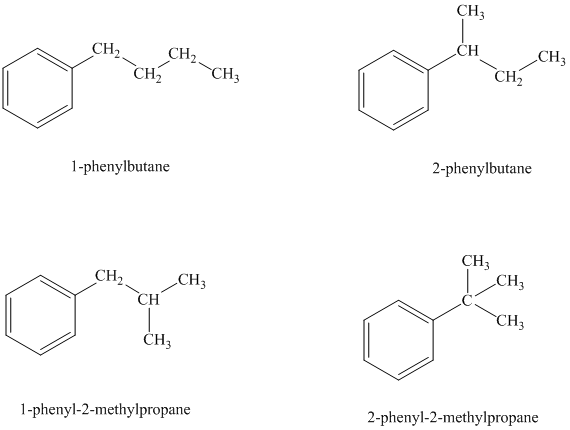
In a mono substituted benzene ring, one hydrogen atom is substituted by a substituent. An alkyl substituent can be arranged such that each carbon atom must have formed four bonds and other atoms if any should have complete octets.
Want to see more full solutions like this?
Chapter 12 Solutions
CAREY: ORGANIC CHEMISTRY
- Don't used hand raiting and don't used Ai solutionarrow_forward2' P17E.6 The oxidation of NO to NO 2 2 NO(g) + O2(g) → 2NO2(g), proceeds by the following mechanism: NO + NO → N₂O₂ k₁ N2O2 NO NO K = N2O2 + O2 → NO2 + NO₂ Ко Verify that application of the steady-state approximation to the intermediate N2O2 results in the rate law d[NO₂] _ 2kk₁[NO][O₂] = dt k+k₁₂[O₂]arrow_forwardPLEASE ANSWER BOTH i) and ii) !!!!arrow_forward
- E17E.2(a) The following mechanism has been proposed for the decomposition of ozone in the atmosphere: 03 → 0₂+0 k₁ O₁₂+0 → 03 K →> 2 k₁ Show that if the third step is rate limiting, then the rate law for the decomposition of O3 is second-order in O3 and of order −1 in O̟.arrow_forward10.arrow_forwardDon't used Ai solution and don't used hand raitingarrow_forward
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning





