![OWLv2 for Ebbing/Gammon's General Chemistry, 11th Edition, [Instant Access], 1 term (6 months)](https://s3.amazonaws.com/compass-isbn-assets/textbook_empty_images/large_textbook_empty.svg)
OWLv2 for Ebbing/Gammon's General Chemistry, 11th Edition, [Instant Access], 1 term (6 months)
11th Edition
ISBN: 9781305673939
Author: Darrell Ebbing; Steven D. Gammon
Publisher: Cengage Learning US
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 1, Problem 1.101QP
Analyses of several samples of a material containing only iron and oxygen gave the following results. Could this material be a compound?
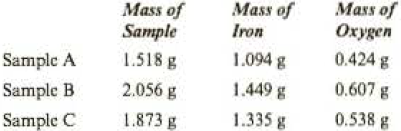
Expert Solution & Answer
Trending nowThis is a popular solution!

Chapter 1 Solutions
OWLv2 for Ebbing/Gammon's General Chemistry, 11th Edition, [Instant Access], 1 term (6 months)
Ch. 1.3 - You place 1.85 grams of wood in a vessel with 9.45...Ch. 1.4 - Potassium is a soft, silvery-colored metal that...Ch. 1.4 - Matter can be represented as being composed of...Ch. 1.5 - Give answers to the following arithmetic setups....Ch. 1.5 - a. When you report your weight to someone, how...Ch. 1.6 - Express the following quantities using an SI...Ch. 1.6 - a. A person with a fever has a temperature of...Ch. 1.6 - Prob. 1.3CCCh. 1.7 - A piece of metal wire has a volume of 20.2 cm3 and...Ch. 1.7 - Ethanol (grain alcohol) has a density of 0.789...
Ch. 1.7 - You are working in the office of a precious metals...Ch. 1.8 - The oxygen molecule (the smallest particle of...Ch. 1.8 - A large crystal is constructed by stacking small,...Ch. 1.8 - Using the definitions 1 in. = 2.54 cm and 1 yd =...Ch. 1 - Discuss some ways in which chemistry has changed...Ch. 1 - Define the terms experiment and theory. How are...Ch. 1 - Illustrate the steps in the scientific method...Ch. 1 - Define the terms matter and mass. What is the...Ch. 1 - Prob. 1.5QPCh. 1 - Prob. 1.6QPCh. 1 - Characterize gases, liquids, and solids in terms...Ch. 1 - Prob. 1.8QPCh. 1 - Give examples of an element, a compound, a...Ch. 1 - What phases or states of matter are present in a...Ch. 1 - What distinguishes an element from a compound? Can...Ch. 1 - Prob. 1.12QPCh. 1 - Prob. 1.13QPCh. 1 - Prob. 1.14QPCh. 1 - How does the International System (SI) obtain...Ch. 1 - Prob. 1.16QPCh. 1 - Prob. 1.17QPCh. 1 - Why should units be carried along with numbers in...Ch. 1 - When the quantity 12.9 g is added to 2 1002 g,...Ch. 1 - Prob. 1.20QPCh. 1 - A 75.0-g sample of a pure liquid, liquid A, with a...Ch. 1 - Which of the following represents the smallest...Ch. 1 - Physical and Chemical Changes Say you are...Ch. 1 - a Sodium metal is partially melted. What are the...Ch. 1 - A material is believed to be a compound. Suppose...Ch. 1 - You need a thermometer that is accurate to 5C to...Ch. 1 - Imagine that you get the chance to shoot five...Ch. 1 - Say you live in a climate where the temperature...Ch. 1 - You are presented with a piece of metal in a jar....Ch. 1 - You have two identical boxes with interior...Ch. 1 - Consider the following compounds and their...Ch. 1 - Which of the following items have a mass of about...Ch. 1 - What is the length of the nail reported to the...Ch. 1 - For these questions, be sure to apply the rules...Ch. 1 - You are teaching a class of second graders some...Ch. 1 - A 15.5 g sample of sodium carbonate is added to a...Ch. 1 - Some iron wire weighing 5.6 g is placed in a...Ch. 1 - Zinc metal reacts with yellow crystals of sulfur...Ch. 1 - Aluminum metal reacts with bromine, a red-brown...Ch. 1 - Give the normal state (solid, liquid, or gas) of...Ch. 1 - Give the normal state (solid, liquid, or gas) of...Ch. 1 - Which of the following are physical changes and...Ch. 1 - For each of the following, decide whether a...Ch. 1 - A sample of mercury(II) oxide was heated to...Ch. 1 - Solid iodine, contaminated with salt, was heated...Ch. 1 - The following are properties of substances. Decide...Ch. 1 - Decide whether each of the following is a physical...Ch. 1 - Iodine is a solid having somewhat lustrous,...Ch. 1 - Mercury(II) oxide is an orange-red solid with a...Ch. 1 - Consider the following separations of materials....Ch. 1 - All of the following processes involve a...Ch. 1 - Label each of the following as a substance, a...Ch. 1 - Indicate whether each of the following materials...Ch. 1 - Which of the following are pure substances and...Ch. 1 - Which of the following are pure substances and...Ch. 1 - How many significant figures are there in each of...Ch. 1 - How many significant figures are there in each of...Ch. 1 - The circumference of the earth at the equator is...Ch. 1 - The astronomical unit equals the mean distance...Ch. 1 - Assuming all numbers are measured quantities, do...Ch. 1 - Assuming all numbers are measured quantities, do...Ch. 1 - Prob. 1.63QPCh. 1 - Prob. 1.64QPCh. 1 - Write the following measurements, without...Ch. 1 - Write the following measurements, without...Ch. 1 - Using scientific notation, convert: a 6.15 ps to s...Ch. 1 - Using scientific notation, convert: a 6.20 km to m...Ch. 1 - Convert: a 68F to degrees Celsius b 23F to degrees...Ch. 1 - Convert: a 51F to degrees Celsius b 11F to degrees...Ch. 1 - Salt and ice are stirred together to give a...Ch. 1 - Prob. 1.72QPCh. 1 - A certain sample of the mineral galena (lead...Ch. 1 - A flask contains a 30.0 mL sample of acetone (nail...Ch. 1 - A liquid with a volume of 8.5 mL has a mass of...Ch. 1 - Prob. 1.76QPCh. 1 - Platinum has a density of 21.4 g/cm3. What is the...Ch. 1 - What is the mass of a 43.8-mL sample of gasoline,...Ch. 1 - Ethanol has a density of 0.789 g/cm3. What volume...Ch. 1 - Bromine is a red-brown liquid with a density of...Ch. 1 - Sodium hydrogen carbonate, known commercially as...Ch. 1 - The acidic constituent in vinegar is acetic acid....Ch. 1 - The different colors of light have different...Ch. 1 - Water consists of molecules (groups of atoms). A...Ch. 1 - The total amount of fresh water on earth is...Ch. 1 - A submicroscopic particle suspended in a solution...Ch. 1 - How many grams are there in 3.58 short tons? Note...Ch. 1 - Prob. 1.88QPCh. 1 - The first measurement of sea depth was made in...Ch. 1 - The estimated amount of recoverable oil from the...Ch. 1 - A fish tank is 24.2 in. long, 15.9 in. deep, and...Ch. 1 - The population density of worms in a particular...Ch. 1 - Prob. 1.93QPCh. 1 - An antacid tablet weighing 0.853 g contained...Ch. 1 - When a mixture of aluminum powder and iron(III)...Ch. 1 - When chlorine gas is bubbled into a solution of...Ch. 1 - A beaker weighed 50.90 g. To the beaker was added...Ch. 1 - Prob. 1.98QPCh. 1 - Describe each of the following as a physical or...Ch. 1 - Describe each of the following as a physical or...Ch. 1 - Analyses of several samples of a material...Ch. 1 - A red-orange solid contains only mercury and...Ch. 1 - A cubic box measures 39.3 cm on an edge. What is...Ch. 1 - A cylinder with circular cross section has a...Ch. 1 - An aquarium has a rectangular cross section that...Ch. 1 - A spherical tank has a radius of 175.0 in....Ch. 1 - Obtain the difference in volume between two...Ch. 1 - What is the difference in surface area between two...Ch. 1 - Perform the following arithmetic setups and...Ch. 1 - Prob. 1.110QPCh. 1 - For each of the following, write the measurement...Ch. 1 - For each of the following, write the measurement...Ch. 1 - Write each of the following in terms of the SI...Ch. 1 - Write each of the following in terms of the SI...Ch. 1 - Tungsten metal, which is used in lightbulb...Ch. 1 - Titanium metal is used in aerospace alloys to add...Ch. 1 - Calcium carbonate, a white powder used in...Ch. 1 - Prob. 1.118QPCh. 1 - Gallium metal can be melted by the heat of ones...Ch. 1 - Mercury metal is liquid at normal temperatures but...Ch. 1 - Zinc metal can be purified by distillation...Ch. 1 - Iodine is a bluish-black solid. It forms a...Ch. 1 - An aluminum alloy used in the construction of...Ch. 1 - Vanadium metal is added to steel to impart...Ch. 1 - The density of quartz mineral was determined by...Ch. 1 - Prob. 1.126QPCh. 1 - Some bottles of colorless liquids were being...Ch. 1 - Providing no reaction occurs, a solid will float...Ch. 1 - Platinum metal is used in jewelry; it is also used...Ch. 1 - Ultrapure silicon is used to make solid-state...Ch. 1 - Vinegar contains acetic acid (about 5% by mass)....Ch. 1 - Ethyl acetate has a characteristic fruity odor and...Ch. 1 - Prob. 1.133QPCh. 1 - Prob. 1.134QPCh. 1 - Convert; a 5.91 kg of chrome yellow to milligrams...Ch. 1 - Convert: a 7.19 g of cyanocobalamin (vitamin B12)...Ch. 1 - The largest of the Great Lakes is Lake Superior,...Ch. 1 - The average flow of the Niagara River is 3.50 km3...Ch. 1 - A room measures 10.0 ft 11.0 ft and is 9.0 ft...Ch. 1 - Prob. 1.140QPCh. 1 - The masses of diamonds and gems are measured in...Ch. 1 - One year of world production of gold was 49.6 106...Ch. 1 - Prob. 1.143QPCh. 1 - All good experiments start with a scientific...Ch. 1 - Prob. 1.145QPCh. 1 - Prob. 1.146QPCh. 1 - Prob. 1.147QPCh. 1 - A 33.0-g sample of an unknown liquid at 20.0C is...Ch. 1 - A 124-g sample of a pure liquid, liquid A, with a...Ch. 1 - On a long trip you travel 832 miles in 21 hours....Ch. 1 - The density of lead at 20C is 11.3 g/cm3. Rank the...Ch. 1 - Prob. 1.152QPCh. 1 - Prob. 1.153QPCh. 1 - The density of liquid water at 80C is 972 kg/m3...Ch. 1 - Prob. 1.155QPCh. 1 - At 20C liquid gasoline gas has a density of 0.75...Ch. 1 - The figures below represent a gas trapped in...Ch. 1 - An ice cube measures 3.50 cm on each edge and...Ch. 1 - The total length of all the DNA molecules...Ch. 1 - Prospectors are considering searching for gold on...Ch. 1 - A solution is prepared by dissolving table salt,...Ch. 1 - Water and saline (salt) solution have in common...Ch. 1 - When 11.1 g of marble chips (calcium carbonate) is...Ch. 1 - Zinc ore (zinc sulfide) is treated with sulfuric...Ch. 1 - A steel sphere has a radius of 1.58 in. If this...Ch. 1 - A weather balloon filled with helium has a...Ch. 1 - Prob. 1.167QPCh. 1 - Prob. 1.168QPCh. 1 - A sample of an ethanolwater solution has a volume...Ch. 1 - You have a piece of gold jewelry weighing 9.35 g....Ch. 1 - A sample of vermilion-colored mineral was weighed...Ch. 1 - A sample of a bright blue mineral was weighed in...Ch. 1 - Prob. 1.173QPCh. 1 - An experimenter places a piece of a solid metal...Ch. 1 - Prob. 1.175QPCh. 1 - The expected outcome for the amount of sugar in a...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A red-orange solid contains only mercury and oxygen. Analyses of three different samples gave the following results. Are the data consistent with the hypothesis that the material is a compound?arrow_forwardWhich of the following are compounds, and which are elements? aNa2S bBr2 cPotassium hydroxide dFluorine eCompound or element fCompound or elementarrow_forwardSuppose someone emptied ball bearings into a container of salt. Could you separate the ball bearings from the salt? How? Would your method involve no change, be a physical change, or be a chemical change?arrow_forward
- You receive a mixture of table salt and sand and have to separate the mixture into pure substances. Explain how you would carry out this task. Is your method based on physical or chemical properties? Explain.arrow_forward1f a piece of hard, white blackboard chalk is heated strongly in a flame, the mass of the piece of chalk will decrease, and eventually the chalk will crumble into a white dust. Does this change suggest that the chalk is composed of an element or a compound?arrow_forwardIf we classify substances as ionic, molecular, macromolecular, or metallic, in which if any categories are all the members a. soluble in water? b. electrical conductors in the melt? c. insoluble in all common solvents? d. solids at room temperature?arrow_forward
- Which of the substances below are pure and which are mixtures? Which could be either? Explain your answers.arrow_forwarda Which of the following substances would you expect to be elements and which would you expect to be compounds? 1 calcium carbonate; 2 arsenic; 3 uranium; 4 potassium chloride; 5 chloromethane. b On what general rule do you base your answers to part a? Can you name any exceptions to this general rule?arrow_forwardWhich of the following represent physical properties or changes, and which represent chemical properties or changes? You curl your hair with a curling iron. You curl your hair by getting a “permanent wave” at the hair salon. Ice on your sidewalk melts when you put salt on it. A glass of water evaporates overnight when it is left on the bedside table. Your steak chars if the skillet is too hot. Alcohol feels cool when it is spilled on the skin. Alcohol ignites when a flame is brought near it. Baking powder causes biscuits to rise.arrow_forward
- A material is believed to be a compound. Suppose you have several samples of this material obtained from various places around the world. Comment on what you would expect to find upon observing the melting point and color for each sample. What would you expect to find upon determining the elemental composition for each sample?arrow_forwardFrom the information given, classify each of the pure substances A through D as elements or compounds, or indicate that no such classification is possible because of insufficient information. a. Substance A cannot be broken down into simpler substances by chemical means. b. Substance B cannot be broken down into simpler substances by physical means. c. Substance C readily dissolves in water. d. Substance D readily reacts with the element chlorine.arrow_forwardWhich of the following is not characteristic of a pure substance? (a) Has a uniform composition throughout (b) Has a fixed melting temperature (c) Can be separated into two other pure substancesarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co
Types of Matter: Elements, Compounds and Mixtures; Author: Professor Dave Explains;https://www.youtube.com/watch?v=dggHWvFJ8Xs;License: Standard YouTube License, CC-BY